Abstract
BACKGROUND
Diffuse large B-cell lymphoma (DLBCL) is a high-grade malignancy and the most common subtype of lymphoma worldwide. Standard therapy with anthracycline-based chemotherapy and rituximab is able to cure about two thirds of patients but a significant proportion will develop relapsed/refractory disease. Salvage treatment options in this setting are limited and only effective in a minority of patients. Most of these patients will eventually die from progressive disease, underscoring the pressing clinical need for the development of new treatment strategies.
Checkpoint blockade therapy has demonstrated efficacy in a variety of cancers and involves modulating the host's immune response towards an effective anti-tumoral attack. Trials using programmed cell death protein 1 (PD-1) blockers in advanced hematological malignancies demonstrated remarkable activity in patients with Hodgkin lymphoma. Unfortunately, results in DLBCL were marginal and short lived. The mechanisms underlying the failure of immune checkpoint blockade in DLBCL are not known. To better understand mechanisms of immune evasion we utilized mass cytometry to the study of immune subsets in the tumor microenvironment (TME) of DLBCL.
METHODS
Viably cryopreserved cell suspensions created from clinical specimens of patients with DLBCL were obtained from our biorepository. Specimens were stained using a cocktail of metal-tagged antibodies recognizing over 32 surface proteins and acquired on the mass cytometer (CyTOF2™, Fluidigm). Control tissue consisted of reactive lymph node (LN) tissue, tonsil and spleen, obtained from patients without diagnosis of cancer. Analysis was performed using Cytobank (www.cytobank.com). Institutional Review Board approved the conduct of this study.
RESULTS
Thirteen patient samples were available for analysis (7 DLBCL, 6 controls). DLBCL samples originated from LN (4), spleen (2), and adrenal gland (1). Control tissue consisted of tonsil (3), reactive LN (2), and normal spleen (1).
Global analysis of CD45+ cells showed that T-cells were more commonly found in the TME of DLBCL than controls (DLBCL 47.2% vs. 24.5% in controls; Figure 1). The difference persisted when the analysis was restricted to LN specimens (50.2% in DLBCL vs. 25.1% in normal LN). On both DLBCL and control samples the majority of T-cells were CD4+ (61.1% in DLBCL; 75.3% in control) compared to CD8+ T-cells (33.3% in DLBCL; 20% in control). DLBCL samples had increased proportion of regulatory T-cells (Treg) compared to controls (16.9% vs. 6.5%), even after stratifying for tissue of origin (Figure 2). Multiparametric analysis of cytotoxic (CD8+) T-cell subsets revealed that majority of CD8+ T-cells in DLBCL has an effector memory (Tem) phenotype (81.9%) compared to tissue-matched control (43%). PD-1 expression was present in the majority of CD8+ T-cells and mapped to the Effector Memory and Effector subsets. The majority of PD-1 positive cells co-expressed CD27 (>80%). One of the samples (DLBCL arising in the spleen from CLL background) also demonstrated bright PD-1 expression in B-cells (Figure 3).
CONCLUSIONS
Mass cytometry reveals that the tumor microenvironment of DLBCL is enriched with CD4+ T-cells including a higher proportion of Treg compared to tissue-matched control. We also demonstrated that most cytotoxic T-cells in the TME of DLBCL have an effector memory phenotype and express other markers of activation and prior antigenic exposure. Co-expression of PD-1 and CD27 was common in CD8+ T-cells, providing rationale for studies of combination immunotherapy. PD-1 expression in B-cells of DLBCL arising from CLL background deserves further investigation.
Proportion of major immune subsets found in the TME of DLBCL (blue) and control tissue (red).
Proportion of major immune subsets found in the TME of DLBCL (blue) and control tissue (red).
Proportion of regulatory T-cells (Treg) found in the TME of DLBCL (blue) compared to control (red). Bars represent standard error.
Proportion of regulatory T-cells (Treg) found in the TME of DLBCL (blue) compared to control (red). Bars represent standard error.
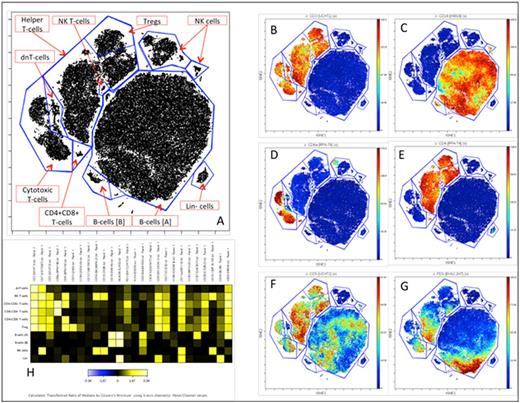
viSNE map of CD45+ cells from a patient with DLBCL arising from a CLL background. Each unit on the dot plot represents a cell. Their coordinates on the map are functions of their phenotypic distance. Major subsets were identified using unbiased clustering and annotated (A). Events were color coded according to the expression of representative markers (B-G). A heat map (H) displays median intensity of expression of a comprehensive set of markers included in the panel for each immune subset.
viSNE map of CD45+ cells from a patient with DLBCL arising from a CLL background. Each unit on the dot plot represents a cell. Their coordinates on the map are functions of their phenotypic distance. Major subsets were identified using unbiased clustering and annotated (A). Events were color coded according to the expression of representative markers (B-G). A heat map (H) displays median intensity of expression of a comprehensive set of markers included in the panel for each immune subset.
Ansell:BMS, Seattle Genetics, Merck, Celldex and Affimed: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.