Abstract
(INTRODUCTION) Several clinical studies have revealed that dasatinib demonstrated deep and fast responses. We report a phase II study to evaluate the efficacy and safety of dasatinib in patients with newly diagnosed chronic-phase chronic myeloid leukemia (CML-CP) in Japan (IMIDAS PART2 study).
(PATIRNTS AND METHODS) Between July 2011 and June 2013, a total of 79 consecutive patients with CML-CP received 100 mg dasatinib daily as a first-line therapy. Treatment was continued until disease progression or until toxicity became unacceptable. The primary end-point was the rate of major molecular response by 12 months. Secondary end-points included the rate of complete cytogenetic response, rate of molecular responses with a 4.5 log reduction (MR4.5) by 12 months, and adverse events. The median age was 62 years, ranging from 27 to 80 years. Patients older than 65 years comprised 41.7% of all patients. Two-thirds of patients (68.4%) were male. Nearly all patients were ECOG performance status 0. Most patients (83.6%) had low and intermediate Sokal scores. The BCR-ABL1 International Scale (BCR-ABL1 IS) in the peripheral blood was measured by the central laboratory center (BML, Tokyo, Japan).
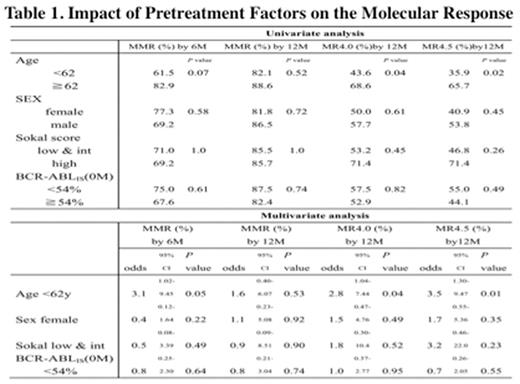
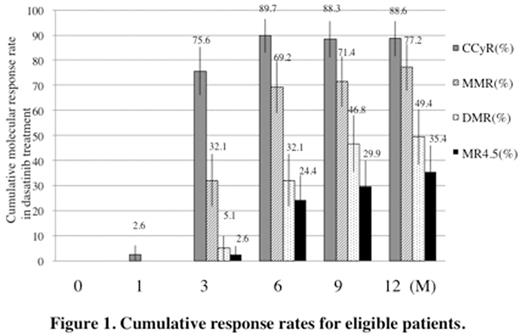
(RESULTS) The median BCR-ABL1 IS before therapy was 54.0% (7.8-230.2). Seventy patients (88.6%) received dasatinib therapy for 12 months. The median BCR-ABL1 ISs were 0.25 % at 3 months (range 0.0002-52.2 %, n =77), 0.03 % at 6 months (range not detected-29.2 %, n = 75) and 0.008 % at 12 months (not detected -0.86 %, n = 70). MMR rate was 77.2% (95% CI, 67.9-86.5 %) by 12 months. The rates of CCyR and MR4.5 by 12 months were 88.6% (95% CI; 81.5-95.7 %) and 35.4% (95% CI; 24.8-46.1 %), respectively (Figure 1). Multivariate analysis of MMR or MR4.5 by 12 months showed that female sex (odds ratio 1.1, P = 0.92, odds ratio 1.7, P = 0.35, respectively), low and intermediate Sokal score (odds ratio 0.9, P = 0.90, odds ratio 3.2, P =0.23, respectively), and BCR-ABL1 IS less than 54% at diagnosis (RR =odds ratio 0.8, P = 0.74, odds ratio 0.7, P = 0.55, respectively) were not significantly correlated with MMR by 12 months nor MR4.5 by 12 months (Table 1). However, patients who were more than 62 years old were significantly correlated with MR4.0 and MR4.5 by 12 months (odds ratio 2.8, P =0.04, odds ratio 3.5, P =0.01). Treatment-related all AEs were reported in 98.7% patients (78 of 79). Grade 3/4 non-hematologic AEs were observed in only a few cases. Lymphocytosis (more than 4x 109/L) was observed in 34.1% of patients, which was within grade 2. Only 9 patients withdrew the study because of adverse events (4 patients), ineffectiveness (3 patients), and others (2 patients).
(DISCUSSION AND CONCLUSION) The incidence of lymphocyte predominance may depend on a history of previous cytomegalovirus (CMV) infection in CML-CP patients (Leukemia. 2011 25(10): 1587-97). Although the frequency of CMV-positive patients was unknown in this study, that of blood donors in Japan was almost positive in the ages with 60s or older. The median age of CML-CP patients in this study was 62 years. Therefore, we assumed that some immunological effects induced by dasatinib might have improved the clinical efficacy in Japanese CML-CP patients compared to those worldwide. Our phase II study to evaluate the efficacy and safety of dasatinib in patients with newly diagnosed CML-CP in Japan revealed that the first-line dasatinib treatment of CML-CP leads to earlier achievement of MMR and MR4.5 with high safety. Elder age patients (>62 years) were significantly associated with achievement of MR4.5.
Shindo:Sysmex Corporation: Research Funding. Sakamoto:Yakult: Other: Remuneration; Takeda Pharmaceutical: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.