Abstract
Ibrutinib is a promising targeted therapy for chronic lymphocytic leukemia (CLL). However, a small subset of patients progress on ibrutinib either through progressive CLL or Richter's transformation. Patients responding to ibrutinib and then progressing with Richter's transformation do so most commonly within the first 2 years of treatment and have an extremely poor prognosis. Identifying biomarkers associated with this transformation is of utmost importance. Near-tetraploidy (4 copies of most chromosomes within a cell) has been reported in various lymphomas; however, its incidence in CLL has not been described. We investigated the prevalence of near-tetraploidy in CLL patients prior to starting ibrutinib and identified it as a pre-treatment biomarker for Richter's transformation.
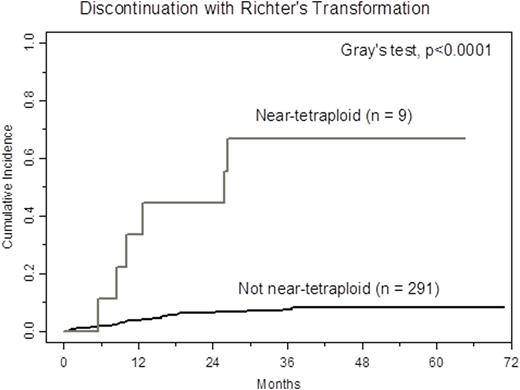
We examined near-tetraploidy in a large series of CLL patients enrolled across four ibrutinib clinical trials at the Ohio State University, for which extensive correlative studies and follow up data are available (previously described by Maddocks et al., JAMA Oncol, 2015). We identified this abnormality in 9 of 300 patients (3.0%, 95% CI: 1.4-5.6) in blood or bone marrow samples taken prior to starting therapy. Near-tetraploidy was detected by the presence of four signals with four or more fluorescence in situ hybridization (FISH) probes and confirmed in the metaphase karyotype of each patient in at least one cell. Near-tetraploidy was associated with aggressive disease characteristics including: Rai stage 3/4 (p=0.03), deletion 17p (p=0.03), and complex karyotype (p=0.01), as well as trisomy 12 (p=0.05). With a median follow-up time of 40.5 months, in patients positive with near-tetraploidy, one patient (11%) progressed with CLL on ibrutinib, six patients (67%) progressed with Richter's transformation, and two patients (22%) were still on treatment. Cumulative incidence of Richter's transformation was significantly higher in patients with near-tetraploidy (Figure; p<0.0001). Notably, near-tetraploidy was not associated with progression with CLL alone (p=0.53). In a multivariable model, both near-tetraploidy (HR 8.66, 95% CI 3.83-19.59, p<0.0001) and complex karyotype (HR 4.78, 95% CI 1.42-15.94, p=0.01) were independent risk factors for discontinuing ibrutinib due to Richter's transformation.
Our results suggest that near-tetraploidy is a distinct biomarker to assess in patients initiating ibrutinib which would predict a high risk for Richter's transformation. As a biomarker it will be important to confirm this association in a second independent data set as well as interrogate the distinct pathophysiology of this genomic subset of CLL.
Lozanski:Stemline Therapeutics Inc.: Research Funding; Boehringer Ingelheim: Research Funding; Genentech: Research Funding; Beckman Coulter: Research Funding. Jones:Pharmacyclics, LLC, an AbbVie Company: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Andritsos:Hairy Cell Leukemia Foundation: Research Funding. Awan:Novartis Oncology: Consultancy; Pharmacyclics: Consultancy; Innate Pharma: Research Funding. Blum:Pharmacyclics: Research Funding. Woyach:Acerta: Research Funding; Morphosys: Research Funding; Karyopharm: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.