Abstract
INTRODUCTION
Minimal residual disease (MRD) assessment is an essential prognosis factor in multiple myeloma (MM). In this way different high-sensitivity quantification methods are being developed and improved using molecular or flow-cytometry approaches. Currently, the only method available to study molecular response by NGS in MM is clonoSEQ; this is offered as external service by Adaptative Technologies. We have optimized a simplified method to quantify molecular response using NGS. In this work, we compare results when quantifying molecular response by our simplified NGS method or by clonoSEQ. Also we study the prognostic impact of molecular response assessed by NGS techniques in a large series of 181 cases.
METHODS
We evaluated the molecular response in 71 patients included in GEM2010MAS65 clinical trial by our simplified method, and in 110 patients included in GEM2005 clinical trial by clonoSEQ. Immunoglobulin clonotype quantification by our simplified method: DNA was amplified in accordance with the terms of BIOMED-2 Concerted Action BMH4-CT98-3936. Standard kits were used to prepare libraries (Life Technologies or New England Biolabs), and sequencing was performed with Ion Torrent™ PGM, Ion S5, or MiSeq systems. The resulting FASTQ were analyzed with specific bioinformatic applications in order to identify and quantify clonal specific sequences (clonotype) present in every proband.
RESULTS
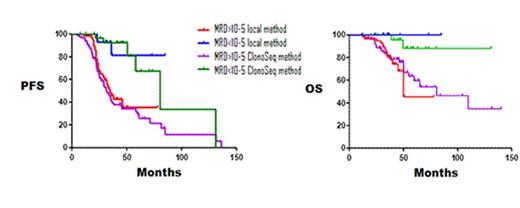
MRD negativity showed significant longer Progression Free Survival (PFS), regardless of the method employed (median 34 months for local method and 32 months for clonoSEQ method in MRD+ patients (MRD>10-5) vs median not reached and 81 months in MRD- patients, respectively, p=0.0001). Likewise, significant differences were observed in terms of Overall Survival (OS) between patients with MRD+ (median 81 months for local method vs 50 months for clonoSEQ method) and MRD- patients (median not reached for both methods), p=0.014. No significant differences were observed in OS and PFS between both local and clonoSEQ NGS techniques (Figure 1).
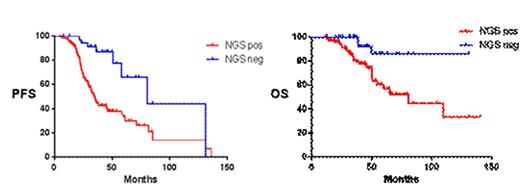
Then, we performed a global analysis with all patients to assess the potential of molecular response evaluated by any NGS method to predict survival regardless of treatment, Molecular response by SEQ was achieved in 43 cases out of 181 (23.7%). Median PFS were 34 vs 80 months for MRD+ and MRD- patients, respectively (p<0.0001, HR=2.8). Median OS were not reached in MRD- patients vs 81 months in MRD+ patients (p=0.004, HR=2.78) (Figure 2).
Regression analysis did not show association between any biological variable and MRD- achievement by NGS. When analyzing MRD- patients, no significant differences in PFS or OS were detected between high or standard-risk cytogenetics cases, comparing to differences observed in overall series or in the MRD+ patients group.
CONCLUSION
Molecular response assessed by NGS is able to identify patients with higher risk, independently of the treatment administered and the method employed. MRD negativity achievement by NGS is an independent risk factor to other clinical and biological variables. Our simplified NGS method offers molecular response information with prognostic value similar to standardized molecular techniques.
Paiva:Celgene: Honoraria, Research Funding; Janssen: Honoraria; Takeda: Honoraria, Research Funding; Sanofi: Consultancy, Research Funding; EngMab: Research Funding; Amgen: Honoraria; Binding Site: Research Funding. Martínez-López:Novartis: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.