Abstract
Background: Elotuzumab is an immunostimulatory monoclonal antibody that binds to signaling lymphocytic activation molecule family member 7 (SLAMF7), which is expressed on myeloma and natural killer (NK) cells. Elotuzumab has a dual mode of action: directly activating NK cells and tagging myeloma cells, thereby initiating antibody-dependent cell-mediated cytotoxicity. In the phase 3 ELOQUENT-2 (NCT01239797) trial involving patients (pts) with relapsed/refractory multiple myeloma (RRMM), elotuzumab in combination with lenalidomide/dexamethasone (ELd) was compared with lenalidomide/dexamethasone alone (Ld) and demonstrated improved progression-free survival (PFS; hazard ratio [HR] 0.70, p<0.001) and overall response rate (ORR; 79% vs 66%, p<0.001) (Lonial S et al. N Engl J Med 2015;373:621-31). We assessed the safety and efficacy of elotuzumab in the ELOQUENT-2 Japanese subpopulation.
Methods: ELOQUENT-2 was a phase 3, open-label, multicenter trial that enrolled pts with RRMM and 1-3 prior therapies. Pts were randomized 1:1 to elotuzumab (10 mg/kg) plus Ld or Ld in 28-day cycles until disease progression, unacceptable toxicity, or withdrawal of consent. All pts received premedication before elotuzumab administration to mitigate infusion reactions. Coprimary endpoints were PFS and ORR. Secondary endpoints and exploratory objectives included overall survival (OS) and safety, respectively.
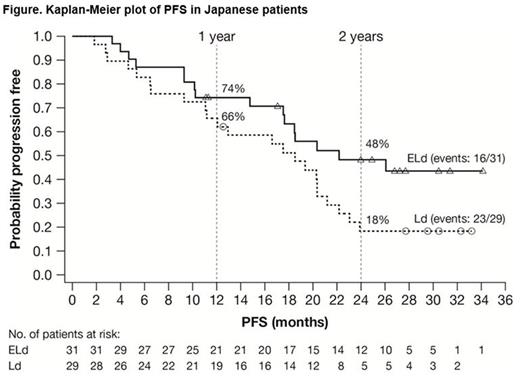
Results: ELOQUENT-2 included 60 Japanese pts in the all-randomized population of 646: 31/321 ELd and 29/325 Ld. The median age was 69 years (range 45-80) in the ELd group and 66 years (range 47-84) in the Ld group. Forty percent (24/60) of pts were refractory to their most recent line of therapy. The median duration of therapy was 22 cycles (range 2-38) in ELd group and 16 cycles (range 3-36) in the Ld group. The data cut-off excluding OS was October 2014; OS was updated October 2015. PFS (ELd vs Ld) was 74% (95% confidence interval [CI]: 55-86) vs 66% (95% CI: 45-80) at 1 year, and 48% (95% CI: 29-65) vs 18% (95% CI: 7-34) at 2 years (Figure). HR for PFS (ELd vs Ld) was 0.51 (95% CI: 0.3-1.1), a 49% reduction in risk of progression/death. Median PFS was 22.2 months (95% CI: 17.5-not estimated [NE]) in the ELd group and 18.5 months (95% CI: 11.1-21.2) in the Ld group. ORR was similar in both groups: 84% (95% CI: 66-95) in the ELd group vs 86% (95% CI: 68-96) in the Ld group. The interim HR for OS between the ELd and Ld groups was 0.81 (95% CI: 0.35-1.87). Survival rates in ELd and Ld pts, respectively, at 1 year were 100% (95% CI: NE-NE) and 97% (95% CI: 78-100); at 2 years, 90% (95% CI: 73-97) and 86% (95% CI 67-94); and at 3 years, 68% (95% CI: 48-81) and 64% (95% CI: 44-79). Grade 3/4 adverse events (AEs) occurring in ≥10% in either groups were neutropenia (ELd 26%, Ld 31%), cataract (ELd 19%, Ld 14%), pneumonia (ELd 19%, Ld 3%), lymphocytopenia (ELd 19%, Ld 3%), decreased appetite (ELd 7%, Ld 14%), anemia (ELd 7%, Ld 10%), hyperglycemia (ELd 7%, Ld 10%), and thrombocytopenia (ELd 3%, Ld 14%). Infections were reported in 81% (25/31) and 79% (23/29) in the ELd and Ld groups, respectively, with exposure-adjusted incidence rates of infection (per 100 person-years) of 172.6 and 183.4, respectively. The incidence of pneumonia was 29% (9/31) vs 7% (2/29) in the ELd and Ld groups, respectively, with exposure-adjusted incidence rates of infection (per 100 person-years) of 16.7 and 4.5, respectively. All pneumonia AEs were reported as serious AEs and were resolved by elotuzumab omission or treatment with antibiotics. No pts discontinued elotuzumab due to pneumonia. Infusion reactions were observed in 4 pts (13%) in the ELd group; all were Grade 1.
Conclusions: In the Japanese ELOQUENT-2 population, elotuzumab showed durable efficacy, with improved PFS and ORR, and with acceptable safety. Incidence of pneumonia tended to be higher with ELd vs Ld in the Japanese subanalysis. All cases were manageable, and none led to treatment discontinuation. Within the constraints of a small subgroup, results were consistent between Japanese pts and the overall pt population.
Study support: Bristol-Myers Squib, Princeton, New Jersey, USA
Sunami:Ono Pharmaceutical: Research Funding; Daiichi Sankyo: Research Funding; Bristol-Myers Squibb K.K.: Research Funding; Novartis: Research Funding; Celgene: Honoraria, Research Funding; Takeda: Research Funding; Sanofi: Research Funding; Janssen Pharmaceutical: Research Funding. Iida:Celgene: Honoraria, Research Funding; Janssen Pharmaceuticals: Honoraria, Research Funding. Okamoto:Nippon Shinyaku Co., Ltd.: Research Funding; Astellas Pharma Inc.: Research Funding; Asahi Kasei Pharma Corp.: Research Funding; Alexion Pharmaceuticals, Inc.: Research Funding; Bristol-Myers Squibb K.K.: Honoraria, Research Funding; Sumitomo Dainippon Pharma Co., Ltd.: Research Funding; Otsuka Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Kyowa Hakko Kirin Co., Ltd.: Research Funding; Teijin Pharma Limited: Research Funding; Chugai Pharmaceutical Co., Ltd.: Research Funding; Toyama Chemical Co., Ltd.: Research Funding; Eisai Co., Ltd.: Research Funding; Shionogi & Co., Ltd.: Research Funding; Pfizer Inc.: Honoraria, Research Funding; JCR Pharmaceuticals Co., Ltd.: Research Funding. Miyoshi:Bristol-Myers Squibb K.K.: Employment. Bleickardt:Bristol-Myers Squibb: Employment. Matsumoto:Celgene: Honoraria; Janssen Pharmaceutical: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.