Abstract
Background
Mixed chimerism, a persistent or increasing number of host cells, after allogeneic hematopoietic stem cell transplant (allo-HSCT) is a predictor of disease relapse. Donor lymphocyte infusion (DLI) has the potential to enhance the graft vs. malignancy effect and reduce the risk of relapse in patients with mixed chimerism (MC) and in patients at high-risk (HR) for relapse. Hence, there is a motivation to utilize DLI for patients with hematologic malignancies while in complete remission (CR) after transplant to prevent disease relapse. To assess the safety and efficacy of DLI after allo-HSCT, in both the relapsed and non-relapsed settings, records of 88 patients receiving DLI between 2003 and 2015 at a single institution were retrospectively reviewed.
Patients & Methods
Median age at time of transplant was 53 (range, 18-68 years). Fifty-four patients received allo-HSCT from related donors (RD) and 34 patients received allo-HSCT from matched unrelated donors (MUD). Reasons for DLI were relapsed/residual disease (n=52, 59%), MC with no evidence of disease (n=29, 33%), and HR with no evidence of disease (n=7, 8%). 44 of 52 relapsed/residual disease patients received treatment before DLI (41 chemotherapy, 4 other, 7 no treatment). 17/52 patients (33%) were treated within 6 months of transplant, the rest after 6 months.DLI was administered according to a dose-escalating protocol in 4-8 week intervals. Starting dose varied based on donor and disease status: 1.0x107 CD3+ cells/kg for relapse/residual RD, 3.0x106 CD3+ cells/kg for relapse/residual MUD, 3.0x106 CD3+ cells/kg for MC/HR RD and 1.0x106 CD3+ cells/kg for MC/HR MUD. Endpoints to discontinue DLI included: full donor chimerism (FDC), remission, GvHD development, disease progression, or lack of further donor cells.
Results
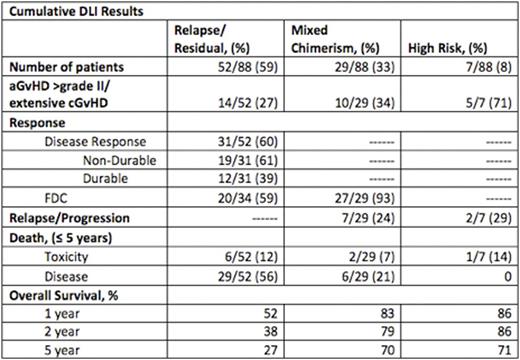
Median interval from transplant to DLI was 195 days (range, 50-2026 days) and median number of DLI infusions was 2 (range, 1-7 infusions). From first DLI, 1 year overall survival (OS) rates were 52%, 83%, and 86% for relapse/residual, MC, and HR populations respectively. 5 year OS rates were 27%, 70%, and 71%, respectively. Cumulative incidence of acute GvHD grade III-IV and extensive chronic GvHD was 33% (29/88 patients). Six patients died of GvHD-related toxicities (7%).
In the relapsed setting, 31/52 patients (60%) responded to DLI with disease control, of whom 12/52 (23%) had durable remissions/disease control while 19/52 (36.5%) eventually relapsed or had disease progression. Thirty-four patients had MC at the time of relapse, and 20/34 patients (59%) returned to FDC after DLI. All 14 patients who did not re-establish FDC after DLI relapsed or had disease progression. Relapse/disease progression remained the most frequent cause of death in the relapsed/residual population, 56%. The 1-year OS was 29% for those who relapsed within 6 months of transplant, 64% for those who relapsed beyond 6 months. The 5 year OS was 18% for those relapsed within 6 months, 33% for those who relapsed beyond 6 months.
DLI induced FDC in 27/29 patients (93%) in the MC population, with durable FDC and ongoing CR in 22/29 patients (76%). Seven MC patients (24%) relapsed after DLI. In the HR population, DLI was protective against relapse in 5 patients while 2 patients (29%) relapsed after DLI.
Conclusions
Ninety three percent of MC patients in the non-relapsed setting achieved FDC after DLI, with a 24% relapse rate over five years. Further, pre-emptive DLI in patients with MC or HR is associated with a 70% 5 year OS. These results suggest a clinically meaningful effect of pre-emptive and prophylactic DLI in MC and HR patient populations. For patients with MC, both relapsed and non-relapsed, achieving FDC after DLI is essential for disease control. All 17 patients in both populations that did not achieve FDC had non-durable responses or no responses to DLI with subsequent disease progression. Fifteen of the 17 patients who did not achieve FDC after DLI died due to disease progression, while the remaining 2 underwent a second allo-HSCT. DLI for HR was associated with significant rates of acute >grade II and extensive chronic GvHD. In this population, development of GvHD may not be the optimal endpoint, in order to limit GvHD-attributable mortality.
Akard:Ariad, BMS, Celgene, Gilead, Novartis, Takeda: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.