Abstract
Most secreted proteins are glycosylated on the asparagine (N) residue with the consensus sequence N-X-S/T(X≠Proline).Coagulation factor VIII (FVIII) is heavily N-linked glycosylated with 5 consensus sites outside the B domain. However, the roles of these glycans are not well understood. Meanwhile, missense mutations which could create additional N-linked glycosylation sites have largely not been characterized in hemophilia A patients.
In this study we first expressed individual domains of FVIII and determined that the A2, Cand C2 domains are efficiently secreted. The A1(N42,N239), A3 (N1810)and C1 (N2118)domains are glycosylated, whereas N582 in the A2 domain is not glycosylated. Only one hemophilia A missense mutation, S241C in the A1 domain, was found to abolish the consensus sequence for N-linked glycosylation at N239. We confirmed that the S241C mutant lost one glycan and became unstable inside cells. We also tested the other three glycosylation sites and found that elimination of the N-linked glycan at N2118 (N2118Q mutation) impaired the secretion of the C domain. This defect could not be rescued by adding another N-linked glycan (at N2062) in the C1 domain, indicating that the N2118 glycan is specifically required for the secretion of the C domain.
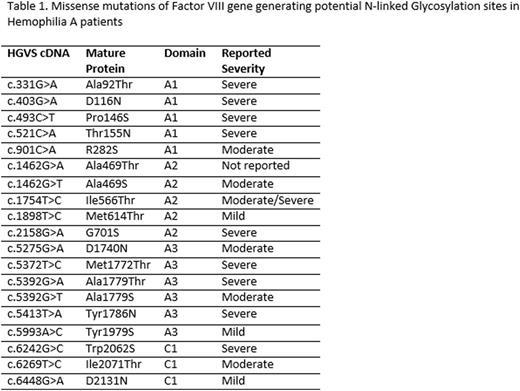
We next searched the CHAMP F8 Mutation Database and the FVIII Variant Database and identified 19 missense mutations that potentially create an ectopic glycosylation site.These mutations are located in A1, A2, A3 and C1 domains, but none in the C2 domain. Only two mutations (I566T and M1772T) have previously been characterized.We found that all but one (I2071T) of these mutations gained an additional N-linked glycan. We further studied missense mutations in the A2 (A469T, A469S, I566T, M614T and G701S) and the C domain (W2062S, I2071T and D2131N) because these domains are secreted in cell culture. Whereas secretion of I566T, W2062S and D2131N mutants was comparable to their wild-type counterparts, secretion of other mutants decreased to 5%-30% of WT (P<0.05). Mutants that secreted into culture media nevertheless have low FVIII activity (<2%), indicating that these mutations cause cross reactive material positive hemophilia A.
The consequences of additional N-linked glycan were further investigated using the A2 domain mutants, since this domain is normally unglycosylated. After treating with tunicamycin to block the N-linked glycosylation process in the endoplasmic reticulum (ER),the secretion of A2 domain with I566T andG701Smutants, which had relatively high secretion levels, decreased significantly. On the other hand, removing the additional glycan boosted the secretion of A469S and A469T, two low-secretion mutants.Tunicamycin treatment had no effect on another low secretion mutant,M614T.These results suggest that amino acid substitution in I566T andG701Smutationsis detrimental to the proper folding of the protein and the additional N-glycan plays a stabilization role. On the other hand, additional N-glycan plays a destabilization role in A469S and A469T mutations, contributing to disruption of folding in these mutants. For theM614Tmutation,the amino acid substitution alone is likely sufficient todestroy the protein folding.
We also studied interactions of abnormally glycosylated mutants with ER chaperones.All the mutants with low secretion levels significantly induced expression of GRP78 to 1.5-2.0 folds(P<0.05), while mutants that maintain higher secretion levels did not affect GRP78 expression. The low secretion mutants also had increased binding to GRP78 and calreticulin, but not to calnexin.Therefore ER chaperones play a key role in the ER quality control of FVIII mutants. In conclusion, our results indicate that the effects of abnormal N-linked glycosylation on FVIII folding and secretionvary widely, from detrimental to beneficial. The impact of a particular glycan is likely determined by the location and the underlying amino acid change caused by the mutation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.