Abstract
Background: Selectins, among other adhesion-mediated functions, facilitate and augment thrombosis; standard anticoagulants address thrombosis but also increase bleeding risk. Previous work in animal models showed inhibiting E-selectin decreases venous thrombosis (VTE) and vein wall inflammation without adverse bleeding events, making E-selectin inhibition a favorable therapeutic candidate for VTE. GMI-1271 is a potent, specific E-selectin antagonist. Here we report final analysis of safety, PK, biomarker and bleeding risk profile for GMI-1271 in 2 phase 1 studies of healthy subjects.
Methods: The first study was a blinded single ascending dose (SAD) evaluation of 2, 5, 20, or 40mg/kg of GMI-1271 (n=4/cohort), vs placebo control saline (n=4) or active control Lovenox 1mg/kg (n=4). The second was an open-label multiple ascending dose (MAD) study of 10 (n=3) or 20mg/kg (n=3) of GMI-1271 QD d 1-5 vs Lovenox 1mg/kg (n=2) d 1-5.
Assessments included safety (adverse events [AEs], clinical labs, bleeding time, PT/PTT, vitals, exam); PK (plasma and urine); and biomarkers. Biomarkers included ELISAs (CRP, D-dimer, IL-10, MPO, Prothrombin fragment 1.2, soluble E-selectin (sEsel), soluble P-selectin (sPsel), sICAM, Thrombin-antithrombin complex (TAT), Tissue Factor (TF), TNFα, VWF activity, and sCD40L); PicoGreen Assay for circulating DNA; flow cytometry (Platelet Monocyte Aggregates (PMA), Mac-1, LFA-1, and CD44) and Thromboelastography (TEG). See Table 1 for functional description.
SAD remains blinded to GMI-1271 or placebo (GMI-1271/p). In SAD, we measured biomarker values at baseline, 8 and 24 h after dosing. Analysis was performed of biomarkers at each dose level and then pooled by GMI-1271/p vs Lovenox. In MAD, we measured biomarker values at baseline and day 4. Comparisons were made using unpaired t-test.
Results: In total, 32 subjects enrolled and received GMI-1271/placebo (GMI-1271/p; 20), GMI-1271 (6) or Lovenox (L; 6).
Safety: All subjects completed dosing uneventfully. Safety findings for GMI-1271/p were unremarkable. No moderate or serious AE were seen. AE overall were as expected in healthy volunteers. In SAD, only 1/20 GMI-1271/p subjects experienced an AE possibly related to study drug (mild transient headache); 0/6 in MAD. In the L group we saw expected mild transaminitis and injection site bruising. In all studies, GMI-1271 had no effect on bleeding time, PT, or PTT.
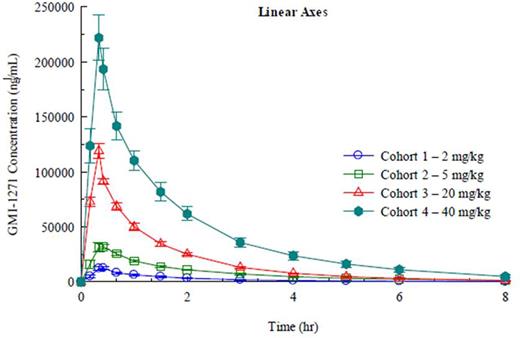
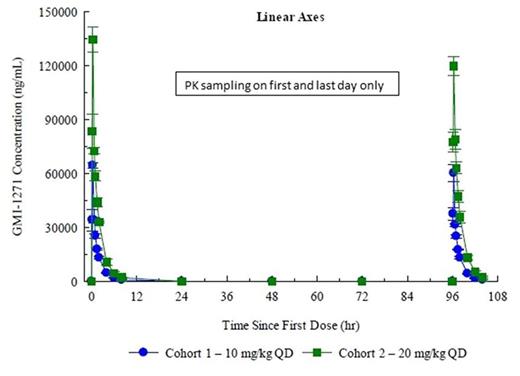
PK: Plasma levels, Cmax, and AUC increased in a linear manner. Cl, Vz, and t ½ were not dose dependent; no accumulation was seen with multiple dosing (Fig 1 and 2).
TEG: In SAD, there was a tendency to increase R, K, and decrease A and MA with no change in % lysis in L vs GMI-1271/p. In MAD, we saw a similar trend as SAD except for an increase in % lysis in L. In the pooled SAD analysis, there was a statistically significant difference between GMI-1271/p and L (higher values) for R (p<0.001) and K (p<0.001); MA was statistically higher in GMI-1271/p vs L (p<0.05).
sEsel: In SAD, there was a trend for sEsel to decrease with treatment in all cohorts (combined GMI-1271/p vs L p= 0.017). In MAD, there was non-significant trend for sEsel to decrease between Day 0 and Day 4 in GMI-1271; sEsel levels trended upward with L in comparison.
MPO: In SAD, there was a trend for increased values in L vs GMI-1271/p, except for the 40mg/kg cohort. When pooled, there was a significant difference between GMI-1271/p vs L (higher levels) p<0.01. In MAD, there was a non-significant trend for higher levels in L vs GMI-1271.
MAC-1 In SAD, there was no change. In MAD, there was a significant decrease in MAC-1 at the 10mg/kg dose (p<0.01).
No other notable trend was seen in the other biomarkers measured.
Conclusion: We report a favorable safety, biomarker and bleeding profile for the E-selectin antagonist GMI-1271 in healthy subjects. There is no signal to suggest GMI-1271 increases bleeding potential based on adverse events, PT, PTT, bleeding time, and TEG values, unlike traditional anticoagulants. An additional unblinded analysis of the biomarkers will be presented at ASH. We note a trend for sE-sel to decrease with GMI-1271 treatment even in this healthy volunteer population, consistent with previous experience and as expected based on mechanism of action. A phase 1 study of GMI-1271 is currently ongoing to evaluate the safety and efficacy of E-selectin antagonism for the treatment of patients with calf vein DVT.
PK Figures: Figure 1: SAD; Figure 2: MAD.
Hemmer:GlycoMimetics, Inc.: Employment, Equity Ownership. Flanner:GlycoMimetics, Inc.: Employment. Parker:GlycoMimetics, Inc.: Employment. Li:GlycoMimetics, Inc.: Employment, Equity Ownership. Froehlich:Novartis: Consultancy; Janssen: Consultancy; Pfizer: Membership on an entity's Board of Directors or advisory committees; Boehringer-Ingelheim: Membership on an entity's Board of Directors or advisory committees; Fibromuscular Disease Society of America: Research Funding; Blue Cross/Blue Shield of Michigan: Research Funding; Merck: Consultancy. Magnani:GlycoMimetics: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Thackray:GlycoMimetics: Employment, Equity Ownership. Sood:Bayer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.