Abstract
Introduction
Asparaginase is a critical component in the treatment of acute lymphoblastic leukemia (ALL). COG AALL07P4 evaluated the pharmacokinetic (PK) and pharmacodynamic comparability of pegaspargase (ASP) and calaspargase pegol (CASP) as part of an augmented BFM chemotherapy regimen for the treatment of newly-diagnosed, NCI high-risk, B-precursor ALL (Angiolillo, JCO 2014). Both ASP and CASP consist of E. coli L-asparaginase linked to polyethelene glycol (PEG) utilizing a succinimidyl succinate or succinimidyl carbamate linker, respectively. Antibodies to either asparaginase or PEG have been associated with rapid clearance of asparaginase.
Methods
Of the 166 patients randomized, 1 was inevaluable; 54 patients were randomized to ASP at 2500 IU/m2,43 to CASP at 2500 IU/m2 and 69 toCASP at 2100 IU/m2; both CASP treatment groups were pooled for this report. Blinded analyses of anti-ASP and anti-CASP antibodies were performed on samples obtained prior to asparaginase administration and at protocol specified time-points through the second cycle of maintenance therapy inpatients withadequatesamples (50 and 111 patients in the ASP and CASP treatment groups, respectively). Immunogenicity assessment included the detection of binding anti-ASP or anti-CASP antibodies by a validated direct enzyme-linked immunosorbent assay (ELISA). All detected antibodies were analyzed in a neutralizing anti-ASP/anti-CASP antibody assay for their neutralizing capacity using a validated enzymatic coupled activity assay. Analysis of anti-PEG antibodies using ELISA was assessed retrospectively, in a subset of patients who had available specimens, as this had not been part of the initial trial design, in 26 and 42 patients in the ASP and CASP treatment groups, respectively, including all patients with anti-ASP/anti-CASP antibody positive samples. All grades of anaphylactic reactions and hypersensitivity were reported according to the CTCAE v3.0 prior to July 2011 and converted into v4.0 codes with subsequent AEs collected in v4.0 beginning with the first asparaginase dose until 60 days after the last dose of asparaginase or being switched to erwinia asparaginase.
Results
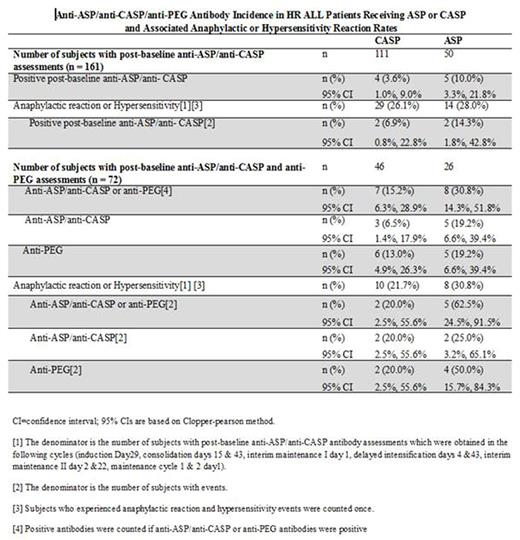
Our analysis of anti-ASP and anti-CASP antibodies showed fewer anti-CASP antibodies: 3.6% (4 /111 patients) than anti-ASP antibodies: 10.0% (5/50 patients). The majority of positive post treatment antibodies were transient and not detectable at subsequent visits. Three patients (one in the CASP and two in the ASP treatment group) showed positive results for binding antibodies at the last tested time point; it is unknown whether these antibodies are of transient or persistent nature. Among 111 patients treated with CASP, 29 (26.1%) developed an anaphylactic and/or hypersensitivity reaction and 2 of these (6.9%) had anti-CASP present at the time of reaction; among 50 patients treated with ASP, 14 (28%) developed an anaphylactic and/or hypersensitivity reaction and 2 of these (14.3%) had anti-ASP present at the time of reaction. Only one patient in the CASP treatment group tested positive for neutralizing antibodies at one time point with negative results at subsequent time points. Focusing on the subset of patients who had available anti-PEG antibody data, we found that 6/46 (13%) in the CASP treatment group had detectable anti-PEG antibodies as compared to the 5/26 (19.2%) in the ASP treatment group. In the patients who had anaphylactic and hypersensitivity reactions, 2/10 (20%) patients treated with CASP had either anti-CASP or anti-PEG antibodies, as compared to 5/8 (62.5%) patients treated with ASP who had either anti-ASP or anti-PEG antibodies. Observed differences in antibody responses were not statistically significant. As we previously reported, many patients with clinical anaphylactic and hypersensitivity reactions did not have antibodies detected.
Conclusions
In this preliminary analysis of COG AALL07P4, patients receiving CASP have numerically less antibody formation to both asparaginase and PEG than patients receiving ASP. Fewer patients with anaphylactic and hypersensitivity reactions to CASP had detectable antibodies as compared to those who received ASP. Since these antibodies may be associated with rapid clearance of asparaginase, further evaluation of PK and immunogenicity is ongoing. Additional investigation is warranted to better understand the clinical significance of these findings.
Schore:Onyx/Amgen: Research Funding; Millennium Pharmaceuticals, Inc: Research Funding; Baxalta: Honoraria; Merck: Research Funding. Bleyer:Jazz Pharmaceuticals: Honoraria, Speakers Bureau. Lebedinsky:Shire: Employment. Zhang:Shire: Employment. Koppensteiner:Shire: Employment. Loh:Abbvie: Research Funding; Briston Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Hunger:Erytech: Honoraria; Patent: Patents & Royalties: Dr. Hunger is a co-inventor of a patent (#8658,964) for the identification of novel subgroups in high risk B-ALL and outcome correlations and diagnostic methods related to the same; Spectrum Pharmaceuticals: Honoraria; Sigma Tau Pharmaceuticals: Honoraria; Jazz Pharmaceuticals: Honoraria; Merck: Equity Ownership; Pfizer: Equity Ownership; Amgen: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.