Abstract
Background: Intercellular adhesion molecule-1 (ICAM-1) is a cell-surface receptor involving in cell-to-cell adhesion and as well as co-stimulatory molecule involved in T-cell lymphocyte infiltration and activation. In the pre-rituximab era, high level of ICAM-1 was reported to be a prognostic indicator of better clinical outcomes in diffuse large B-cell lymphoma (DLBCL). The value of ICAM-1 expression in the post-rituximab era remains unclear. To investigate the impact of ICAM-1 expression in DLBCL, we conducted a retrospective study in R+CHOP treated patients.
Methods: Patients (N=102) with histologically proven DLBCL treated at Fudan University Shanghai Cancer Center with available diagnostic biopsy material were identified. Forty-three patients were treated with CHOP and 59 patients with R+CHOP. ICAM-1 expression was determined by immunohistochemistry (IHC). ICAM-1 expression was quantified by staining intensity or percentage of positive cells. Tumors were considered positive when at least 75% of tumor cells expressed ICAM-1. Progression free survival (PFS) and overall survival (OS) was plotted by Kaplan-Meier method and curves were compared with the long-rank test in both groups. Correlation between the ICAM-1 expression and clinical variables were tested by Pearson Chi Square test and a two-sided P value of <0.05 was considered to be statistically significant. Finally, ICAM-1 expression was determined in a panel of rituximab-sensitive (RSCL) and rituximab-resistant lymphoma cell lines (RRCL) by western blot. Correlation of ICAM-1 expression and rituximab anti-tumor activity was determined by standard complement-mediated cytotoxicity (CMC) and antibody-dependent cellular cytotoxicity (ADCC) assays.
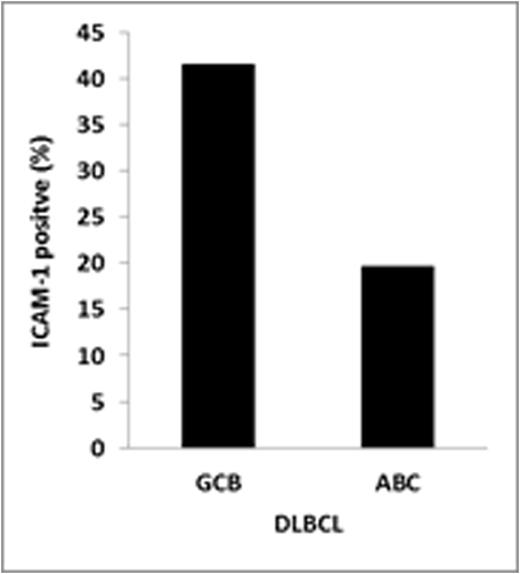
Result: ICAM-1 expressionwas detected in 28 (27.5%) DLBCL patients. The rest of the patients tested negative for ICAM-1 (N=74; 72.5%). Differences in ICAM-1 expression were observed according to cell of origin DLBCL subtype. ICAM-1 expression was higher among germinal center B-cell (GCB) DLBCL (41.4%) vs. non-GCB subtype patients (19.6%, P=0.019). The response rates for R-CHOP and CHOP were, respectively, 89.4% and 77.7% (P=0.409) in ICAM-1 positive patients and 92.5% and 73.5% (p=0.027) in ICAM-1 negative patients. As previously described, ICAM-1 expression was associated with an improved PFS/OS (P=0.03/ P=0.05) in CHOP-treated DLBCL. On the other hand, no differences in PFS/OS were observed between ICAM-1 positive or negative DLBCL patients treated with R+CHOP. The addition of rituximab to CHOP chemotherapy resulted in an improved PFS in ICAM-1 negative DLBCL (P=0.018). On the other hand, the clinical outcome of ICAM-1 positive DLBCL patients treated with CHOP chemotherapy was similar than R+CHOP treated patients. Finally, loss of ICAM-1 expression level was found in our rituximab resistant cell lines, along with reduction of rituximab activity.
Conclusion: Before therituximab era, ICAM-1 low expression correlated with worse PFS and OS. In our retrospective clinical analysis, we found that rituximab significantly improved the overall response rate and PFS in ICAM-1 negative subset patients. Our data also indicated that ICAM-1 expression level was higher in GCB than non-GCB subtypes, and this may contribute to better rituximab response in GCB cell types. Gradually exposed lymphoma cell to rituximab lost ICAM-1 expression and may contribute to the resistance to rituximab and chemotherapy agents. Further investigation of ICAM-1 expression needed to be study to enhance the rituximab therapy in lymphoma.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.