Abstract
Background-
High grade B-cell NHL's are 200 fold more common in seropositive as compared to seronegative patients. It includes diffuse large B cell lymphoma (DLBCL) (50%), Burkitt's (BL)(40%) and others such as plasmablastic lymphoma(PBL). They are biologically different from their seronegative counterparts with higher incidence of extranodal involvement, advanced stage and poor response to conventional therapy. In addition, they are at a higher risk of chemotherapy side effects and poorly tolerate dose intense regimens. To add to the complexity, social stigma and financial constraints decrease the compliance to therapy. R-EPOCH is designed to provide a balance between efficacy and safety. We report on our cohort of patients who were treated with this protocol.
Methods-
We retrospectively analysed HIV-associated de-novo high grade B-cell NHL patients who were treated with R EPOCH regimen at Tata Memorial Centre from 2011 till 2015. Patients aged ≥18 years who had received at least 1 cycle were included in the analysis. R EPOCH is an infusional regimen in which the dose of cyclophosphamide is adjusted depending on the baseline CD4+ T cell count and tolerance. The primary objective was the 3-year overall survival(OS), secondary objectives were response rates, the incidence of grade3/4 toxicities, dose intensity and correlation of OS with CD4 count, IPI, duration of HIV, Cyclophosphamide dose intensity (CDI). Demographic features, HIV related details (CD4 count at diagnosis, ART regimen), histological diagnosis, stage, bulky disease, extranodal sites, treatment details (number of cycles, dose administered), response, toxicity and status at last follow up were recorded. Descriptive statistics were summarised with median and range, survival outcomes were analysed with Kaplan meier method and impact of CD 4 count, CDI, IPI and duration of HIV on survival was assessed using log rank test.
Results-
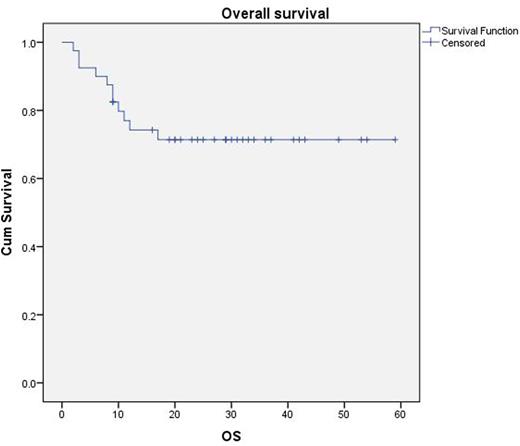
A total of 40 patients(31males) with a median age-40 years (24-65 years) were treated. B symptoms were present in 19(48%). The cohort comprised of DLBCL-19 (48%), BL-16(40%), High grade B-Cell Lymphoma-Unclassifiable-4 (10%) and PBL 1 (2.5%). 16 (40%) patients had co-morbidities, including co-existent Hepatitis C in 5 and Hepatitis B in 2. HIV infection was detected at the time of lymphoma diagnosis in 18 (45%). The median CD4+ T cell count was 202 cells/mm3, 6 patients (15%)had count of <100/mm3 All patients received HAART while on chemotherapy, NNRTI plus 2 NRTI combination being the commonest regimen-33(82%) patients. Performance status (ECOG) ≤2 was seen in 36(90%) patients. 38 (95%) had stage III/IV disease and bulky disease (defined as size ≥7cm) was seen in 29(72%) patients. Extranodal involvement was seen in 36(90%) patients. Haemoglobin level ≤11g/dL in 10(25%) patients (range- 8.2-15.6 g/dL), serum albumin <4g/dL in 23(58%) patients, serum LDH ≥ upper normal limit -38 (95%)patients. Atleast 4 cycles of chemotherapy were administered to 35 (93%)patients, with 28 (70%) receiving 6 cycles. Cyclophosphamide dose escalation was possible in 18(45%) patients. CNS prophylaxis was administered with intrathecal methotrexate (median number-6) to 36(90%) patients. High dose methotrexate was given to 5 patients. Rituximab was not administered to 5 patients (3 due to CD4 count <100, 2 due to CD20 negativity). Responses were assessed using 18FDG PET-CT scan with complete response-32(80%), partial response-1(3%), disease progression-4(10%) and not evaluable- 4 (7%). Grade ¾ toxicities were seen in 33(83%) patients, with febrile neutropenia-26(65%), mucositis-10(25%) and peripheral neuropathy in5(13%) patients. Out of the 210 cycles administered, there were 41(20%) episodes of hospitalisation (duration ranged from 2-51 days). There were 11(28%) deaths (progression-7, toxicity-2, Not known-2) and 7(18%) patients had progression (4- on treatment progression, 3- relapses). With a median follow-up of 29 months, estimated 3-year OS is 71% (Figure 1) and 3-year progression free survival is 79%. There was no difference in survival based on IPI, CD 4+ T cell count, CDI or duration of HIV.
Conclusions-
R-EPOCH is a highly effective regimen in seropositive high-grade B-cell lymphoma even in the presence of adverse features (advanced stage, extranodal sites, CNS involvement, low CD4+ T cell count). The emphasis should now be on further reducing the toxicities.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.