Abstract
Background
The Dynamic International Prognostic Scoring System (DIPSS)-plus currently provides the most comprehensive prognostic model for primary myelofibrosis (PMF) (JCO 2011;29:392). DIPSS-pluse uses eight independent predictors of inferior survival: age >65 years, hemoglobin <10 g/dL, leukocyte count >25 x 109/L, circulating blasts ³1%, constitutional symptoms, unfavorable karyotype, red cell transfusion need and platelet count <100 x 109/L. More recently, type 1/type 1-like CALR driver mutational status and ASXL1 andSRSF2 mutations were identified as additional DIPSS-plus-independent risk factors for survival in PMF (JAMAOncol. 2015;1:97). Based on our earlier preliminary observation (Leuk Res. 2007;31:1503), we conducted the current study to fully examine the additional prognostic relevance of monocytosis (absolute monocyte count ≥1 x 10(9)/L) in PMF.
Methods
Study patients fulfilled the 2016 WHO criteria for the diagnosis of PMF, including both pre-PMF and overt PMF (Blood. 2016;127:2391). Additional selection criteria included the availability of absolute monocyte count (AMC) and percentage at time of referral. Targeted next generation sequencing was used to screen for prognostically-relevant mutations (Blood 2015 126:354). Statistical analyses considered clinical and laboratory parameters obtained at time of initial referral to the Mayo Clinic.
Results
Patient characteristics:
The study population included 454 patients (median age 64 years; 62% males); 293 (64%) patients harbored JAK2 mutations, 74 (16%) type 1/like CALR, 16 (3%) type 2/like CALR, 24 (5%) MPL and 47 (10%) were "triple-negative". DIPSS-plus risk distribution was 29% high, 38% intermediate-2, 17% intermediate-1 and 15% low; 27% displayed red cell transfusion-dependency, 29% constitutional symptoms, 70% palpable splenomegaly and 36% abnormal karyotype, including 13% with unfavorable karyotype. 301 patients were screened for ASXL1 mutations with 39% mutated, 297 for SRSF2 mutations with 15% mutated, 286 for U2AF1 with 18% mutated, 253 for SF3B1 with 6% mutated, 229 for EZH2 with 5% mutated and 110 for IDH1/2 with 7% mutated.
Clinical, cytogenetic and molecular comparisons between PMF patients with and without monocytosis:
Among all study patients, 376 (83%) displayed AMC <1 x 10(9)/L; the remaining had either moderate (AMC between 1 and 3 x 10(9)/L; n=65) or marked (AMC >3 x 10(9)/L; n=13) monocytosis. Comparison of these three groups revealed that monocytosis was significantly associated with older age (p=0.0001), higher leukocyte count (p<0.0001), lower platelet count (p=0.01), higher circulating blast percentage (p=0.0002) and higher DIPSS-plus risk distribution (p=0.01). There were no significant differences in the distribution of driver mutations, ASXL1, SRSF2, U2AF, SF3B1, EZH2 or IDH1/2 mutations or incidence of unfavorable karyotype.
Survival analysis:
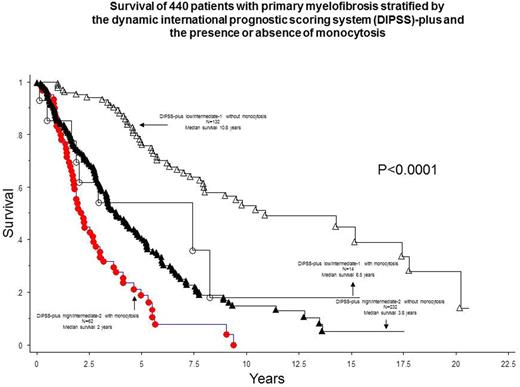
After a median follow up of 3.2 years, 265 (58%) deaths and 38 (8%) leukemic transformations were documented. In univariate analysis, all 8 variables included in DIPSS-plus were significantly associated with shortened survival (p<0.001 in all instances). Significant risk factors in univariate analysis also included AMC >3 x 10(9)/L (p<0.0001; HR 5.6, 95% CI 3.1-10.0), AMC 1 to 3 x 10(9)/L (p<0.0001; HR 2.1, 95% CI 1.5-2.9), absence of CALR type 1/type 1-like (p<0.0001) and presence of ASXL1 (p<0.0001) or SRSF2 (p<0.0001) mutations. The significant difference in survival between the three AMC categories (i.e. <1, 1-3 and >3 x 10(9)L) was independent of each one of the eight DIPSS-plus variables, DIPSS-plus, driver mutational status, ASXL1 and SRSF2 mutations (p<0.0001 in all instances). The survival difference between patients with and without monocytosis was evident in both high/intermediate-2 and low/intermediate-1 DIPSS-plus risk groups (Figure). Patients with monocytosis were also more likely to undergo leukemic transformation (HR 3.0, 95% CI 1.4-6.5) and this effect was also independent of karyotype, driver mutational status, ASXL1 or SRSF2 mutations.
Conclusion
The current study identifies monocytosis as a powerful risk factor for both overall and leukemia-free survival in PMF and the effect was independent of currently established prognostic models and genetic risk factors. It was particularly noteworthy to document similar distribution of driver mutations among the three monocyte categories.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.