Abstract
A Phase II study of Tacrolimus and Thymoglobulin, as Graft-Versus-Host-Disease Prophylaxis in Patients Undergoing Related Donor Allogeneic Hematopoietic Cell Transplantation
Introduction: Regimens used for the prevention of acute GVHD in allogeneic transplantation usually consist of combinations of a calcineurin inhibitor with an additional agent such as methotrexate, mycophenolate or sirolimus. Previously, we and others have demonstrated efficacy of the addition of thymoglobulin to these standard GVHD regimens in preventing both acute and chronic GVHD without increasing relapse rates. Given the proven activity of thymoglobulin, we now report the outcomes of a prospective phase II trial testing the combination of tacrolimus and thymoglobulin in the prevention of GVHD in patients undergoing matched related allogeneic transplants.
Patients and Methods: Patients were eligible if they were between ages 18 to 75 years with hematologic malignancies in complete or partial remission at the time of transplant. Patients received thymoglobulin (4.5 mg/kg total dose) in divided doses from day -3 to -1 days prior to transplant. All patients were also given tacrolimus to maintain a therapeutic level from day -3 to day +60. After 60-days post-transplant, tacrolimus was tapered in the absence of GVHD and discontinued by day + 181. Primary objective was to determine incidence and severity of acute GVHD (aGVHD). Secondary objectives were to determine neutrophil and platelet engraftment time, estimate incidence of chronic GVHD (cGVHD), overall survival (OS), relapse free survival (RFS) and GVHD-free relapse-free survival (GRFS) at 1-year post-transplant. The cumulative incidence of GVHD was calculated using relapse and NRM as competing risks. The OS, RFS and GRFS were reported using KM method. The proportions of incidence of infections were reported with the Wilson's 95% Confidence Interval.
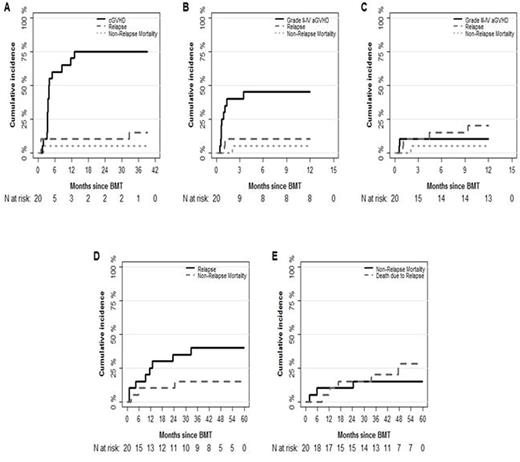
Results: A total 20 patients were enrolled in our study. The median age was 59 years (range, 38-69) and the median follow-up duration was 54.3 months (95% CI, 43.6-NA). Seven patients had MDS, 5 had AML, 4 had NHL, one with CLL, 2 had CML, and one had ALL. Low, intermediate and high disease risk index was noted in 5%, 70%, and 25% of these patients respectively. Conditioning regimens included busulfan (BU)/fludarabine (FLU) (n=7), BEAM (BCNU, etoposide, ara-c and melphalan) ± rituximab (n=4), BU/FLU/total-body irradiation (TBI) (n=7), FLU/melphalan/TBI (n=2). All patients received peripheral blood stem cell transplants (PBSCT). The median day to neutrophil and platelet engraftment was 11 days (range, 7-12) and 17 days (range, 0-25) respectively. The 1-year incidence of grade II-IV aGVHD was 45% (95% CI, 22.3-65.4) and of grade III-IV aGVHD was 10% (95% CI, 1.6%-27.8%). The median time to development of grade II-IV aGVHD was 21 days (range, 14-105). The incidence of cGVHD at 1-year post-transplant was 70% (95% CI, 42.9-86.0) and the median time to development of cGVHD was 114 days (range, 46-616). Fourteen patients (60% with 95% CI, 34.3-78.4) developed extensive cGVHD at 1-year post-transplant. The incidence of relapse and non-relapse mortality at 2-year post-transplant were 35% (95% CI, 15.1-55.9) and 10.0% (95% CI, 1.6-27.8) respectively. Cytomegalovirus (CMV) and Epstein-Barr Virus (EBV) reactivation were noted among 35% and 40% of patients respectively. Eight patients relapsed and the median time of relapse was 317 days (range, 31-994). OS, RFS and GRFS at 1-year post-transplant were 85% (95% CI, 70.7-100), 65% (95% CI, 47.1-89.7) and 25% (95% CI, 11.7-53.4) respectively. Causes of death for 8 patients were: 5 relapse, 2 cGVHD, and 1 multiorgan failure.
Conclusion: We reported a novel GVHD prevention regimen using tacrolimus and thymoglobulin. Our data suggest that combination can be used safely and thymoglobulin may replace either mycophenolate, methotrexate or sirolimus while avoiding some of the toxicities commonly associated with these agents. More importantly, rates of acute and chronic GVHD as well as GRFS appear to be comparable to other GVHD regimens. No significantly increased rates of opportunistic infections were noticed. Further studies in larger patient groups are warranted to better define the role of this regimen in GVHD prevention.
Cumulative incidence of cGVHD, Grade II-IV and III-IV aGVHD, relapse and NRM
Cumulative incidence of cGVHD, Grade II-IV and III-IV aGVHD, relapse and NRM
OS, RFS and GVHD-free relapse-free survival
Deol:Jazz Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.