Abstract
Background: Melphalan at 200mg/m2 (Mel-200) preceding autologous hematopoietic cell transplantation (auto-HCT), is part of the upfront management of patients with multiple myeloma (MM) and has also been used in the salvage setting, often as second transplant. While lenalidomide or bortezomib are routinely employed as maintenance therapy after upfront auto-HCT, there is no established maintenance strategy for patients receiving auto-HCT for relapsed MM. Proteasome inhibitors (PI) have been shown in vitro to impair the Fanconi/BRCA pathway of DNA repair and increase DNA fragmentation and apoptosis induced by alkylating agents in MM cells. Carfilzomib (K), a second generation PI, has been combined with conventional doses of alkylator agents with encouraging efficacy. We hypothesized that K could be safely combined with Mel-200 (K-Mel) in a conditioning regimen prior to auto-HCT and employed as post-transplant maintenance.
Methods: We performed a phase 1, multi-center, dose escalation trial with traditional 3+3 design to determine the maximal tolerated dose (MTD) of K in combination with Mel-200. Subsequently in phase 2, the MTD cohort was expanded for additional safety and efficacy information on the conditioning regimen and to test the safety, efficacy, pharmacodynamics and patient-preference of two distinct schedules of K maintenance. Eligible patients had symptomatic MM, relapsed after at least one line of therapy, with evaluable disease and having obtained at least a minimal response (MR) after the most recent salvage regimen. Patients were required to have ≥2 x 106 CD34+ cells/kg available for transplant. Conditioning consisted of two doses of K administered IV over 30 min. on days -3 and -2. The day -2 dose was administered 1 h prior to administration of Mel-200. K dose consisted of 20(day-3)/27 (day -2) mg/m2 (cohort 0), 27/27 (cohort 1), 27/36 (cohort 2), 27/45 (cohort 3) and 27/56 mg/m2 (cohort 4, phase 2 dose). Maintenance was initiated after 100 days from transplant (phase 2 only). During maintenance patients received K 36 mg/m2 on days 1,8 and 15 (schedule A) or days 1,2,15 and 16 (schedule B) of each 28-day cycle. Each patient receives 2 cycles of each schedule with starting schedule determined by randomization, and 8 subsequent cycles of the patient-preferred schedule.
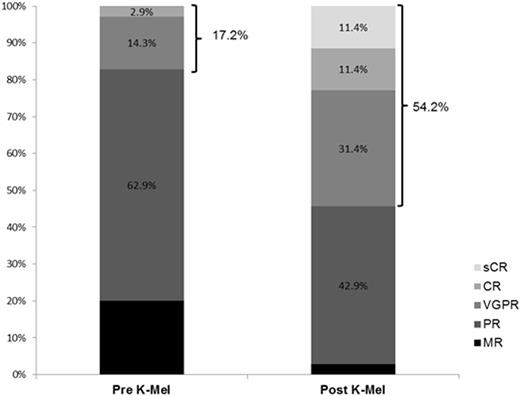
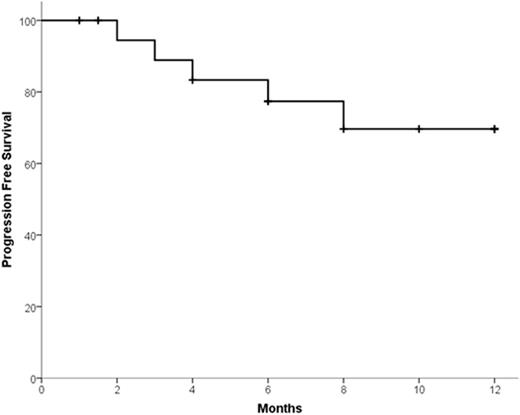
Results: Fifteen patients were treated during phase 1 with no dose-limiting toxicity. Therefore no MTD was reached and the maximal tested dose was employed during phase 2. Accrual is complete with 31 patients treated in phase 2. Overall 46 patients (phase 1 + phase 2) have received K-Mel conditioning. Among all the 46 patients, median age was 58.5 years (range 41-69). Median number of prior lines of therapy was 3 (range 2-6); 98% received prior bortezomib, 93% lenalidomide, 16% pomalidomide, 51% prior carfilzomib, and 58% prior auto-HCT. Pharmacodynamic studies in peripheral blood mononuclear cells revealed that K precluded melphalan-induced increase in FANCD2 and FANCI mRNA. Thirty-five patients (15 phase 1 + 20 phase 2) had 100 days of follow up and underwent disease reassessment, while 11 received K-Mel and auto-HCT but have not yet reached day 100. Median time for engraftment was 10 days (range 8-15) for neutrophils and 16 days (range 9-24) for platelets. There were no non-hematologic grade 4 toxicities after K-Mel. Response categories before and 100 days after K-Mel conditioning are displayed in Figure 1. The proportion of patients with response ≥ VGPR increased from 17.2% before to 54.2% after K-Mel (P=0.003). Twenty patients (phase 2 only) have entered maintenance. Two patients discontinued maintenance due to toxicity (congestive heart failure and acute renal failure) and 5 due to progression. There have been no deaths on study. Interim one-year PFS is 69.6% (Figure 2, 95% C.I. 46.9-92.3%). Among the 16 patients who have completed at least 4 cycles of K maintenance, 9 have choose schedule A and 7 schedule B for subsequent cycles.
Conclusion: K-Mel is a well-tolerated and active conditioning regimen for patients with relapsed MM. K is a feasible and active maintenance agent for patients undergoing auto-HCT for relapsed MM. Updated safety, efficacy, pharmacodynamics and patient preference for maintenance schedule will be presented at the meeting.
Costa:Sanofi: Honoraria, Research Funding. Landau:Spectrum Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Onyx/Amgen: Research Funding; Prothena: Honoraria, Membership on an entity's Board of Directors or advisory committees. Hari:Merck: Research Funding; BMS: Honoraria. Saad:Astellas: Research Funding; American Porphyria foundation: Research Funding; Alexion: Honoraria; Spectrum: Honoraria. Hamadani:Takeda: Research Funding. Petrovic:Amgen: Employment.
Author notes
Asterisk with author names denotes non-ASH members.