Abstract
Introduction:
Acute myeloid leukemia (AML) with chromosomal translocation t(7;11)(p15;p15) [t(7;11)], which results in a fusion between NUP98 and HOXA9, is uncommon, and classified as intermediate risk (IR) in National Comprehensive Cancer Network (NCCN) Guidelines. Previous studies have reported that the patients with AML harboring t(7;11) exhibited a worse clinical outcome than the other AML groups. These reports included mostly patients who underwent chemotherapy rather than allogeneic hematopoietic stem cell transplantation (allo-HSCT), and the number of patients in each study was very small. Moreover, the transplant outcomes of patients with AML harboring t(7;11) compared to patients with other AML have not been reported.
Therefore, we investigated the transplant outcomes among 91 patients with AML harboring t(7;11) compared to the patients with IR (n=7,308) and poor risk (PR) (n=2,406) AML, except for t(7;11). Moreover, we evaluated the risk factors for survival in patients with AML harboring t(7;11) who underwent allo-HSCT.
Patients and Methods:
During 1997 and 2014, 91 patients with AML harboring t(7;11) were identified from the nationwide registration data of the Japan Society for Hematopoietic Cell Transplantation.
Cytogenetic risk group stratification was performed using the NCCN guidelines for AML in 2016.
Univariate and multivariate analysis for survival were performed in all patients cohort and only among patients with AML harboring t(7;11). Univariate models for overall survival (OS) and disease-free survival (DFS) included age at allo-HSCT (age 55 ≥ years vs. 55 < years), sex, performance status (PS) (2-4 vs. 0-1), the hematopoietic cell transplantation-comorbidity index (0-2 vs. ≥ 3), additional chromosomal change, disease status at allo-HSCT (first complete remission [CR]: CR1 vs. second CR:CR2 or non-CR at allo-HSCT), conditioning regimen (total body irradiation [TBI] ≥ 8Gy vs. others), and stem cell source (related donor vs. cord blood or unrelated donor). Factors associated with at least borderline significance (p < 0.20) on univariate analyses were subjected to multivariate analysis.
Results:
Patient Characteristics
We evaluated the clinical characteristics of patients with t(7;11), IR, and PR.The median follow-up period for survivors was 1,124 days (range, 1-8,758). Patients with t(7;11) were younger (median age: 45 vs. 48 vs. 50 years, p<0.01), included more females (49.5 vs. 42.6 vs. 36.2%, p<0.01), and more frequently had PS less than 2 (82.5 vs. 80.2 vs. 76.3%, p<0.01), unrelated donor (40.7 vs. 38.3 vs. 33.9%, p<0.01), received myeloablative TBI (52.4 vs. 42.3 vs. 34.2%, p<0.01), and underwent allo-HSCT at CR1 (48.4 vs. 40.0 vs. 30.9%, p<0.01). Among the patients with t(7;11), 64 (70.3%) had French-American-British classification of M2, and 8 (8.7%) had additional chromosomal change.
Transplantation Outcomes
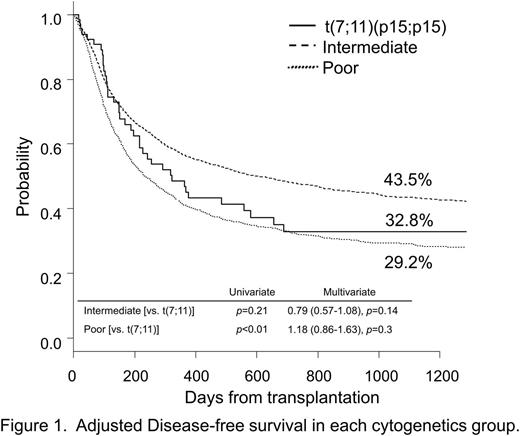
At 3 years after allo-HSCT, OS and DFS in the t(7;11) group were 34.4% and 32.8%, respectively, and tended to be lower than those in the IR group after adjusting background characteristics (47.9% [hazard ratio {HR}=0.79, p=0.15] and 43.5% [HR=0.79, p=0.14]). However, OS and DFS in the t(7;11) group were similar to those in the PR group (33.9% [HR=1.18, p=0.3] and 29.2% [HR=1.18, p=0.27]) (Figure 1).
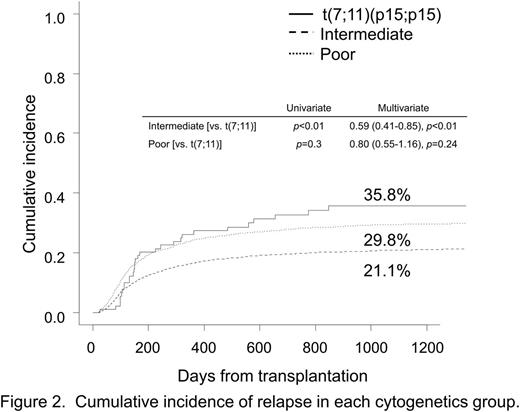
Interestingly, the estimated cumulative incidence of relapse (CIR) at 3 years in the t(7;11) group was higher than that in the IR group (35.8% vs. 21.1% [HR=0.59, p<0.01]), but comparable to that in the PR group (29.8% [HR=0.8, p=0.24]) (Figure 2). In contrast, the estimated cumulative incidence of transplant-related mortality (TRM) in the t(7;11) group was similar to that in the IR/PR group (26.4% vs. 34.2% [HR=1.18, p=0.52] and 43.8% [HR=1.55, p=0.09]).
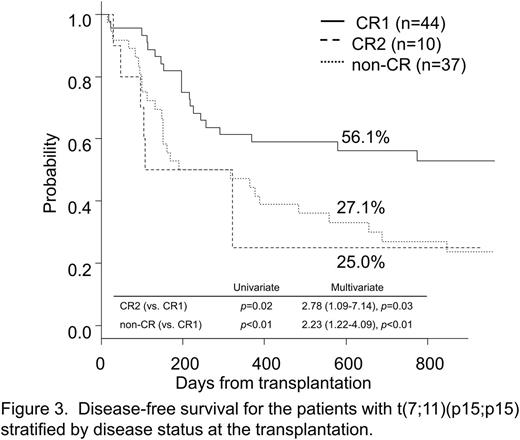
In multivariate analysis among the t(7;11) group, the only factor influencing DFS was disease status (CR2: HR=2.78 [p=0.03], non-CR: HR=2.23 [p<0.01]) (Figure 3). In addition, patients in the CR1 group showed a lower CIR (21.0%) compared with patients in the CR2 or non-CR group (45.0% [HR=2.25, p=0.16], 44.9% [HR=2.31, p=0.03]) at 2 years.
Conclusion:
OS and DFS for the AML with t(7;11) following allo-HSCT were lower than those in the IR group due to higher CIR, but similar to those in the PR group. Among the patients with AML harboring t(7;11), disease status was the only independent prognostic factor for survival in the setting of allo-HSCT. Allo-HSCT at CR1 may overcome the poor prognosis of AML with t(7;11).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.