Abstract
Background:
The myelodysplastic syndromes (MDS) encompass a heterogeneous group of hematopoietic stem cell disorders characterized by dysplastic and ineffective blood cell production leading to cytopenia and a variable risk of transformation to acute myeloid leukemia.
The natural history of patients (pts) with MDS is variable and several prognostic scoring systems have been developed to guide treatment decisions.
The revised IPSS (IPSS-R) score is commonly used in practice to predict outcome in newly diagnosed pts as well as in predicting their transplant outcomes.
It remains unclear however whether improving IPSS-R pre allogeneic transplant (allo-SCT), using different therapeutic strategies, is associated with better clinical outcomes post-transplant.
Methods:
The Leukemia/BMT Program of British Columbia database was queried to identify all pts with MDS who had undergone an allo-SCT between Feb 1997 and April 2013. Pertinent information on clinical features and outcomes were then retrospectively reviewed. IPSS-R was calculated at MDS diagnosis (dx) and then re calculated prior to transplant. Outcomes of pts who had improvement in IPSS-R were then compared to those with no improvement or worsened IPSS-R score. Overall survival (OS) and Event free survival (EFS) were estimated using the Kaplan-Meier method and a competing risk analysis was used to calculate relapse and non-relapse mortality (NRM). Univariate and multivariate analyses were conducted. Log-Rank test was used to determine the p value.
Results:
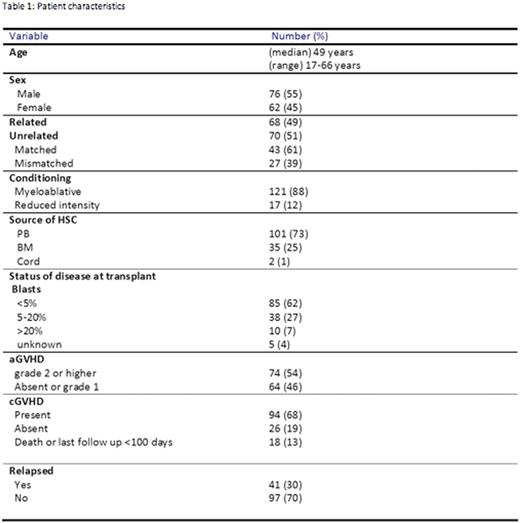
We identified 138 pts who have undergone allo-SCT with the following characteristics: median age at transplant was 49 years (yrs) (range 17-66); 76 (55%) were male; 121 pts (88%) underwent myeloablative (MA) conditioning, 68 (49%) related donor and 70 (51%) unrelated donors out of which 43 (61%) were matched and 27 (39%) were mismatched. The source of stem cells were: peripheral blood n=101 (73%), bone marrow n=35 (25%) and cord blood n=2 (1%). The median interval from dx to transplant was 128 days. The median follow up (FU) of live pts was 7.3 yrs (range 1.5-17.4). Acute graft vs. host disease (aGVHD) grade 2 or higher was present in 74 (54%), chronic graft vs. host disease (cGVHD) was present in 94 (68%). During the time of FU 83 (60%) of pts had died. Relapse occurred in 41 (30%). Causes of death were: relapse n=39; GVHD n=20; regimen related n=11; infection n=5 and other causes, n=9. Baseline characteristics of all pts are shown in table 1. In 12 (9%) pts, the IPSS-R could not be calculated either because cytogenetics failed or bone marrow biopsy pre transplant was not done. At the time of transplant 85 (62%) pts had blasts <5%, 38 (28%) had 5-20% and 10 (7%) had blasts >20% and blasts count was unknown in 5 pts. IPSS-R improved in 62 (45%), worsened in 23 (17%), no change 41 (30%) and unknown in 12 (9%). Type of treatment was chemotherapy in 55 (40%), best supportive care in 80 (58%) and immunosuppressive therapy (IST) in 3 (2%).
The OS and EFS for all pts were 34% and 33%, respectively. There was no difference in outcome between pts who have undergone MA vs non-MA, OS 34% and 39% (p=0.63) and EFS 34% and 32% (p=0.86), respectively.
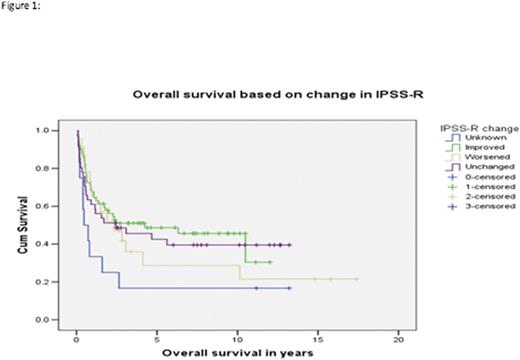
OS was not statistically different between pts with improved IPSS-R vs worsened vs unchanged, 30% vs 22% vs 40%, p= 0.63 (see figure 1). EFS was 30% vs 21% vs 40%, p=0.53, respectively. Relapse was 36% vs 53% vs 31%, p=0.35 and non-relapse mortality was 54% vs 57% vs 42%, p=0.75, respectively. There was no difference in OS and EFS between pts treated with chemotherapy vs supportive care with OS of 40% vs 41%, p=0.63 and EFS of 33% vs 32%, p=0.46, respectively. OS and EFS for grade 2 or higher aGVHD were 34% vs 32%, p=0.13 and 32% vs 32%, p=0.22, respectively. Figure 2 shows OS for Pts with blast <5% vs 5-20% vs >20% at transplant. Relapse of pts with blasts <5 vs 5-20 vs >20 at the time of transplant was 23% vs 69% vs 66%, p=0.0004, respectively. On multivariate analysis, only three factors were associated with worse OS and EFS which were: cytogenetics at dx, blast count at transplant and absence of cGVHD.
Conclusion:
Improving IPSS-R before allogeneic transplant does not translate into better clinical outcome. The IPSS-R cytogenetic risk group at the time of diagnosis and blast count at transplant are highly predictive of post-transplant outcomes.
Patient characteristics
Gerrie:Roche Canada: Research Funding. Toze:Roche Canada: Research Funding. Song:Janssen: Honoraria; Otsuka: Honoraria; Celgene: Honoraria, Research Funding. Song:Janssen: Honoraria; Otsuka: Honoraria; Celgene: Honoraria, Research Funding. Nevill:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.