Abstract
Background:
A cancer diagnosis results in a multitude of health care decisions that require adequate patient understanding to navigate the health care system. Understanding the type and degree of information needs of cancer patients is imperative to instructing effective and pertinent patient education. This study sought to evaluate the impact of a patient centered cancer symposium on knowledge level, knowledge needs and symptom burden in a cancer patient population.
Methods:
Surveys were distributed to attendees of the Mayo Clinic "Living with and Surviving Cancer" patient symposium in January 2016. A follow up survey was sent to participants 3 months after the symposium. Surveys included demographic data, questionnaire evaluating disease comprehension and information assessment, symptom burden, desired information and quality of life assessment. Responses were compared between patients with hematologic and solid tumor malignancies by chi-square test. Pre and post comparisons were done in patients completing both surveys by McNemar test.
Results:
Demographics: 150 patients completed the pre-intervention survey. Average age of participants 67.5 years. Some patients reported more than 1 malignancy. Disease types represented included hematologic malignancies (N=60), specifically multiple myeloma (N=23), lymphoma (N=26), leukemia (N=8) and MPNs (N=3) as well as solid tumor malignancies (N=90). Most patients were diagnosed with cancer at least 1 year prior to the intervention (N=137). Patients with hematologic malignancies were more likely to be on active treatment at the time of the convention (N=35, 58%) compared to solid tumor malignancies (N=37,41%).
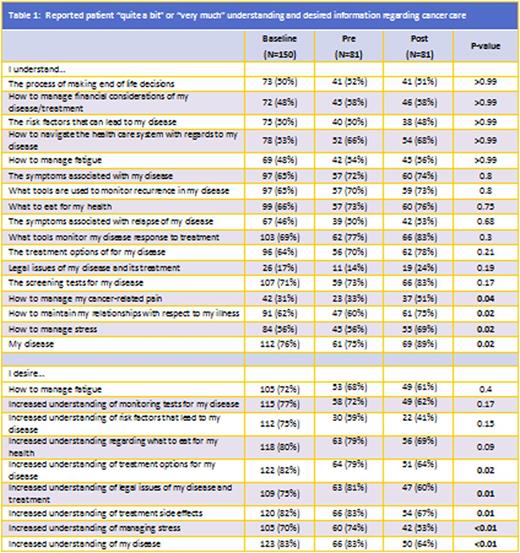
Baseline Knowledge: Most patients reported an adequate level of knowledge defined as the proportion of participants reporting "quite a bit" or "very much understanding" on the topic of disease understanding (76%). Lowest levels of knowledge were legal issues (18%), pain management (31%), disease relapse symptoms(46%), fatigue management (48%), end of life decision making (50%) and financial management (50%).
Patients with hematologic malignancies were more likely to report understanding of fatigue management (N=36, 60%) but less like to report understanding disease risk factors (N=20, 33%), or increased nutritional interest following diagnosis (N=32, 53%) compared to solid tumor malignancy (N=33,37%) (N=55,61%)(N=70,78%), respectively (all p <0.05).
The EORTC-INFO25 at baseline revealed hematologic patients had received less information on sexual side effects of treatments (N=7, 14%) compared to solid tumor patients (N=30, 35%; p=0.004), and reported a lower score of treatment information (50 vs 59; p=0.04).
Pre and Post Analysis: Of the 150 initial participants, 81 completed the pre and post-convention surveys (54%). Post-symposium, significant improvement defined as the proportion of participants reporting "quite a bit" or "very much" understanding was noted of disease (pre 75% vs post 89%), stress management (pre 56% vs post 69%), maintain relationships (pre 60% vs post 75%) and pain management (pre 33% vs post 51%)(p-value<0.05).
The desire for increased information decreased post-symposium regarding all 9 topics, with significant decreases noted in disease, treatment options and side effects, legal issues and stress management.
The EORTC INFO25 analysis revealed a decrease in the reported desire for more information (mean; pre 70 vs post 53, p-value <0.05)
Discussion:
Cancer patients have a variety of information needs. A patient centered educational conference was able to produce significant improvement in understanding in 4 of 17 topics (24%), including general disease, stress and pain management, and relationship maintenance that was durable at 3 months. At least some improvement in understanding was noted in 10 of 17(59%) additional topics. Knowledge deficit improved in all 9 topics assessed, significantly in five. Cancer patient knowledge needs are varied, and likely evolve over time as a patient progress through stages of disease and treatment. Education should re-evaluate and redistribute information to cancer patients over the course of treatment and survivorship.
Mikhael:Abbvie: Research Funding; Celgene: Research Funding; Sanofi: Research Funding; Onyx: Research Funding. Dueck:Bayer: Honoraria. Mesa:Novartis: Consultancy; Ariad: Consultancy; Promedior: Research Funding; Galena: Consultancy; Incyte: Research Funding; Celgene: Research Funding; CTI Biopharma: Research Funding; Gilead: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.