Abstract
Background: Inotuzumab ozogamicin (InO), a humanized anti-CD22 antibody-calicheamicin conjugate, produced a superior response compared with standard of care (SOC) for relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL) in an intent-to-treat (ITT) analysis of the first 218 of 326 patients (pts) randomized (ITT218) in the INO-VATE trial (complete remission [CR], including CR with incomplete hematologic recovery [CRi], 80.7% [95% CI, 72-88] vs 29.4% [21-39]; 1-sided P<0.0001; Kantarjian NEJM 2016). In an ITT survival analysis of all 326 pts randomized (ITT326), progression free survival (PFS) was significantly longer with InO vs SOC (HR, 0.45 [97.5% CI, 0.34‒0.61]; 1-sided P<0.0001; median, 5.0 [95% CI, 3.7‒5.6] vs 1.8 [1.5‒2.2] months [mo]). Overall survival (OS) was longer (HR, 0.77 [97.5% CI, 0.58-1.03]; 1-sided P=0.0203; median, 7.7 [95% CI, 6.0‒9.2] vs 6.7 [4.9‒8.3] mo; 2-year OS rate, 23% [95% CI, 16‒30] vs 10% [5‒16]). Here, outcomes in the ITT326 population by salvage 1 (S1) vs salvage 2 (S2) status are presented.
Methods: In the INO-VATE trial (NCT01564784), adults with CD22-positive ALL due to receive S1 or S2 treatment were randomized (1:1) to InO (starting dose 1.8 mg/m2/cycle [0.8 mg/m2 on day 1; 0.5 mg/m2 on days 8 and 15 of a 21-28 day cycle for ≤6 cycles]) or SOC (either fludarabine/cytarabine [ara-C]/granulocyte colony-stimulating factor [FLAG], ara-C plus mitoxantrone, or high-dose ara-C). Outcomes included investigator assessed CR/CRi, minimal residual disease (MRD)-negativity in responders (assessed by central flow cytometry [threshold <0.01%]), duration of remission (DoR), OS, and PFS. Data as of March 8, 2016 for the final analysis are presented (long-term safety follow-up is ongoing).
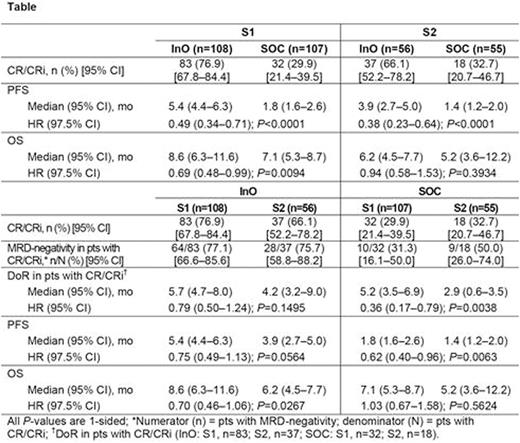
Results: Baseline characteristics for the ITT326 were balanced for S1 vs S2 for InO (S1, n=108; S2, n=56) and SOC (S1, n=107; S2, n=55); except more S2 vs S1 pts were Philadelphia chromosome-positive (InO, 20% vs 10%; SOC, 24% vs 14%), had complex cytogenetics (InO, 30% vs 9%; SOC, 18% vs 11%), and had prestudy SCT (InO, 31% vs 11%; SOC, 28% vs 14%). For InO vs SOC, CR/CRi rates were significantly higher for both S1 and S2 pts (both 1-sided P≤0.0002; Table). For S1 vs S2, CR/CRi rates were numerically higher for the InO arm and were similar for the SOC arm; comparable MRD-negativity rates among responders were reported for the InO and SOC arm. None of these S1 vs S2 differences were significant. For S1 vs S2, DoR was longer for the InO and SOC arms; this difference was significant for the SOC arm.
For InO vs SOC, PFS was significantly longer for both S1 and S2 pts. For this same comparison, OS was significantly longer for S1, but not S2. For S1 vs S2, PFS was numerically longer in both the InO and SOC arms; this difference was significant in the SOC arm. For S1 vs S2, OS was also numerically longer in the InO arm and was similar in the SOC arm.
The most common Grade (Gr) ≥3 adverse events (AEs) in both S1 and S2 pts receiving InO or SOC were cytopenias; Gr ≥3 febrile neutropenia rates were similar for S1 vs S2 for the InO(24% vs 29%) and SOC (56% vs 51%) arms, as were Gr ≥3 infection rates (system-organ class: InO, 28% vs 28%; SOC; 54% vs 57%). For S2 vs S1,Gr ≥3 hepatobiliary AEs rates were higher for the InO (26% vs 12%) arm and were similar for the SOC (9% vs 6%) arm. During InO therapy or during follow-up without intervening SCT, more S2 vs S1 pts had veno-occlusive disease (VOD: 6% [n=3/51] vs 2% [n=2/111]).For InO vs SOC, poststudy SCT rates were significantly higher in both S1 and S2 pts (S1, 51% [56/111] vs 25% [24/95]; S2, 39% [20/51] vs 17% [8/47]; both P≤0.02); VOD rates in pts with poststudy SCT were higher for InO vs SOC (S1, 18% [10/56] vs 4% [1/24]; S2, 35% [7/20] vs 0% [0/8]). More S1 vs S2 pts receiving InO had poststudy SCT (51% [n=56/111] vs 39% [n=20/51]); the VOD rate among pts who receivedpoststudy SCT was greater for S2 (35% [n=7/20]) vs S1 (18% [n=10/56]). Seven of the VOD cases occurring in the InO arm were reversible.Five pts receiving InO had grade 5 VOD (all post-SCT).
Conclusions: InO provides clinical benefit regardless of salvage status for pts with R/R ALL, while OS benefit appeared more evident in S1 pts. However, small sample sizes impose limits on interpretation of these results. InO safety profiles were similar across subgroups, except for the incidence of liver-toxicities, including VOD, which may be greater in heavily pretreated pts such as those in later lines of therapy.
Advani:Blinatumomab: Research Funding; Pfizer Inc.: Consultancy, Research Funding. Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. Stelljes:Pfizer: Consultancy. DeAngelo:Amgen: Consultancy; Novartis: Consultancy; Incyte: Consultancy; Ariad: Consultancy; Celgene: Consultancy; Pfizer: Consultancy; Baxter: Consultancy. Liedtke:Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Stock:Gilead Sciences: Honoraria; Royalties for a chapter in Up to Date: Patents & Royalties; Sigma-Tau: Honoraria, Research Funding; Amgen: Honoraria; ADC Therapeutics: Honoraria. Gökbuget:Pfizer: Honoraria, Research Funding; Amgen: Honoraria, Research Funding. O'Brien:Celgene: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Gilead Sciences: Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Janssen: Honoraria, Other: Travel, Accommodations, Expenses; Pfizer: Honoraria; Pharmacyclics: Honoraria; ProNAi: Honoraria; Regeneron: Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Amgen: Consultancy; CLL Global Research Foundation: Consultancy; Acerta Pharma: Research Funding. Wang:Pfizer: Employment, Equity Ownership. Wang:Pfizer: Employment, Equity Ownership. Paccagnella:Pfizer: Employment. Sleight:Pfizer: Employment, Equity Ownership. Vandendries:Pfizer Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract