Abstract
Introduction
Adults with acute lymphoblastic leukemia (ALL) continue to have worse outcomes when compared to their pediatric counterparts, due to differences in disease biology, comorbidities and ability to tolerate therapy. Patients treated with the hyper-CVAD regimen (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with methotrexate and cytarabine) are reported to have up to a 92% complete remission (CR) rate and 38% 5-year overall survival (OS) rate in clinical trials. Less is known about treatment outcomes and feasibility outside of clinical trials.
Methods
Adult (≥18 years) patients with ALL receiving a hyper-CVAD backbone regimen from January 1st 2005 through May 30th 2016 at the University of California, Davis Comprehensive Cancer Center (UCDCC) were retrospectively identified using a pharmacy-generated electronic medical record report. Baseline patient characteristics, including age, gender, performance status, disease type, and risk features, were collected. All patients received antimicrobial prophylaxis and growth factor support as standard of care. Efficacy was evaluated by CR, 2-year OS, 5-year OS, and time to relapse. Survival analysis was performed using the Kaplan-Meier method. Assessment of toxicity included adverse events, number of cycles, dose modifications, and 30- and 60-day all-cause mortality.
Results
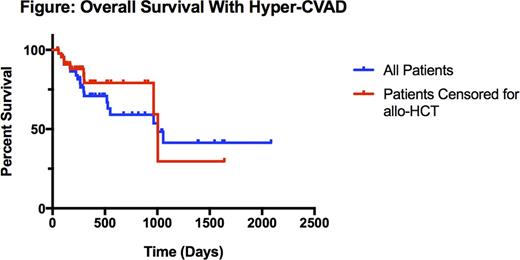
Forty-eight patients were identified. Median age was 48 (range 18-68) years. Eighteen (37.5%) patients were categorized as adolescents and young adults (AYA), defined as age 18 to 39 years. Thirty-two (66.7%) patients were male. Forty-one (85.4%) patients had precursor-B or B-Cell ALL. At diagnosis, 8 patients (16.7%) had central nervous system disease, 9 (18.8%) were Philadelphia Chromosome (Ph)-positive, and 13 (27.1%) had a leukocytosis greater than 30 K/mm3. Patients received a median of 5 (range 1-8) cycles. The median number of cycles received was 5.5 (range 1-8) for AYA patients and 4.0 (range 1-8) for adults. Overall, 37 (77.1%) patients achieved CR and 11 (22.9%) had refractory disease. Two-year OS was 59.1%, 5-year OS was 40.7%, and the median survival was 2.75 years (Figure). Censoring for receipt of allogeneic stem cell transplant (allo-HCT), two-year OS was 79.1% and the median survival was 2.75 years (Figure). Thirteen (28.2%) patients received an allo-HCT. For patients who did not undergo allo-HCT, median survival was 2.64 years. At 2 years, 57.1% of AYA and 37.5% of adult patients were alive. Median time to relapse was 198 (range 86-1212) days. Thirty-day all-cause mortality was 0% and 60-day all-cause mortality was 2%. Twenty-two (45.8%) patients required dose modification.
Conclusions
Outcomes after hyper-CVAD for adult ALL treated at UCDCC are similar to those reported in prior single institution series. Early death rates were low, with 60-day mortality of only 2%, though dose modifications were common. CR rates were lower than expected based on prior reports, potentially related to differences in the populations treated. Despite this, the 2-year OS was 59.1%, likely due to effective salvage in non-responders or early relapses. Hyper-CVAD is an effective and feasible regimen that can serve as a backbone for future clinical trials.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.