Abstract
Introduction
Tacrolimus is a widely used calcineurin inhibitor in solid organ transplantation and in allogeneic HCST for prophylaxis and treatment of GvHD. Recently, several transplantation centers showed advantageous effects for patients with tacrolimus in comparison with cyclosporin A (CsA) regarding renal function after renal and heart transplantation. Based on these observations, we have conducted a prospective study in wich CsA is switched for tacrolimus (Prograf or Advagraf ER) in case of renal impairment due to CsA after allogeneic
HSCT.
Patients, Method
We enrolled 31 consecutive patients between March 2012 and March 2016 from two centers in France (Lyon and Nancy) with renal impairment due to CsA (serum creatinine > 90 µMol/L), the conversion dose was established on an mg:mg basis 1 :100 from CsA total daily dose to a total daily dose of tacrolimus. In this cohort, 27 patients received oral formulation Advagraf and 4 received Prograf initially i.v. and then converted to oral form. The dose was readjusted to obtain a tacrolimus blood trough level between 5 and 15 µg/L.We evaluated the tacrolimus blood trough level changes after conversion, serum creatinine, potassium, one time a week from one week after switching to discontinuation. Before the switch, 22 patients (70%) had CsA for GvHD prophylaxis and 9 patients (30%) for acute GvHD treatment in association with prednisone.
Results
All patients had hematological malignancies, the median age was 54 years (range, 17-67). Twelve patients (39%) had a matched related donor, 19 patients (61%) had a HLA-10/10 matched unrelated donor .The stem cell source was bone marrow for 11 patients (35.5%), PBSC for 18 patients (58%) and cord blood for 2 patients (6.5%). Fifteen patients (48%) received a myeloablative regimen and 16 patients (52%) a reduced intensity regimen. ATG was administrated in 28 patients and 10 patients received 12Gy TBI. The status at transplantation was CR1 for 13 patients (42%), CR2 or more for 10 patients (32%), partial response for 5 patients (16%) and 3 patients (10%) had a refractory leukemia.
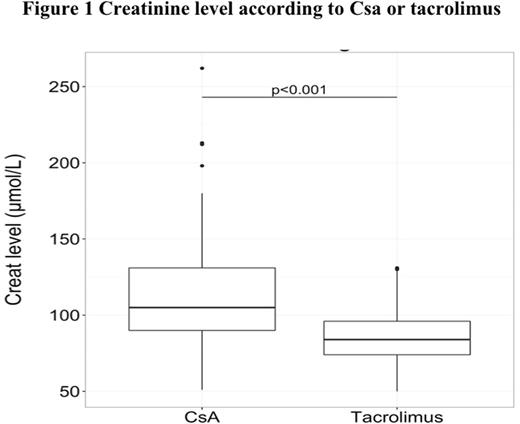
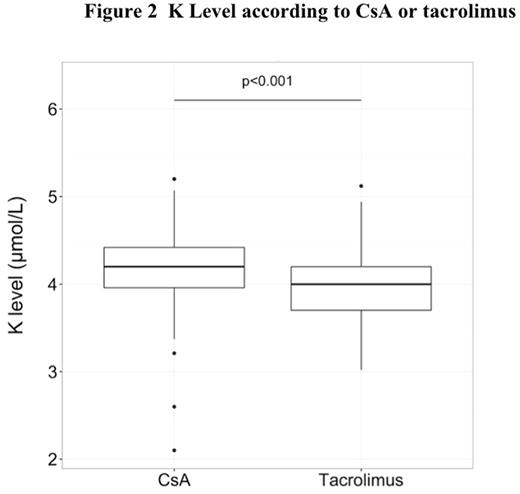
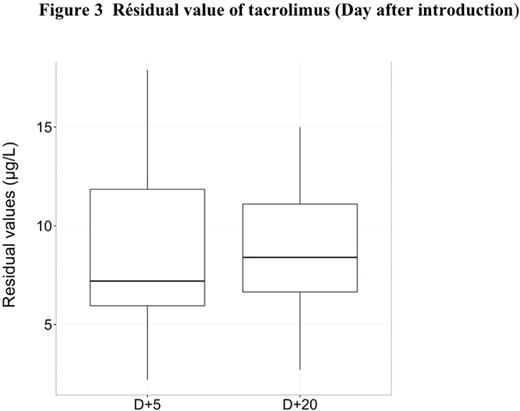
The median follow-up after transplantation was 35.6 months (range, 1-51,7). The median time of switch for tacrolimus was 40 days (range, 3-1286) and the median of serum creatinine in wich the switch was realized was 110 mol/L (range, 94-262). Concerning creatinine level, the median of serum creatinine was 105 µmol/L (range, 51-262) with CsA and 84 µmol/L (range, 50-129) with tacrolimus (p<0.01) (Figure 1). Moreover the median of potassium level was 4.2 mmol/L (range,3.2-5.2 ) with CsA and 4.0 (range,3-5.1 ) with tacrolimus (p<0.001 ) (Figure 2). Before the switch, the median of serum creatinine was 94µMol/L (range, 51-213),106µMol/L (range, 57-262) and 110 µMol/L (range, 75-180) at D+30, D+45 and D+60 respectively after transplantation for patients with CsA and the median residual value of CsA was 250 µg/l (range, 162-629) at D+30. The median dose of CsA was 200mg (range, 25-600) and the median dose of tacrolimus was 2.4mg (range, 0.5-5). The median of serum creatinine was 88µMol/L (range, 59-130), 84µMol/L (range,54-129) and 84µMol/L (range, 54-129) at D+5, D+15 and D+30 after the switch for tacrolimus and the median residual value of tacrolimus was 8.7µg/l (range,2.7-15) at D+20 after the switch (Figure 3).
The cumulative incidence of grade II-IV aGvHD was 53% (95%CI, 25-64) at +90 days. Seventeen patients (58%) developed aGvHD grade ≥ II and 7 patients (22%) developed chronic GvHD with NIH score 2-3 after discontinuation of GvHD prophylaxis. The cumulative incidence of cGvHD was 26.5% (95%CI, 7.75-45) at 1 year, 32.14%(95% CI, 11.6-52) after 2 years and 32.14% (95% CI, 11.6-52) after 3 years. In this cohort, 27 patients (87%) are alive and 26 patients (84%) are in complete response at this time. Four patients died two from relapse, one from cerebral bleeding and one from severe pneumonia (fungal and Pneumocystis carinii infection).
Conclusion
The conversion from CsA to Tacrolimus was followed by a clinically significant improvement in kidney function with stable tacrolimus blood trough levels. Based on these observations, we suggested that the use of tacrolimus in case of renal impairment due to CsA is safe in allogeneic HSCT patients.
Michallet:Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Astellas Pharma: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Genzyme: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract