Abstract
Allogeneic HCT is a crucial tool for the treatment of hematologic malignancies, immune-deficiencies, and metabolic disorders. Donor-derived T-cells are critical for the graft-versus-leukemia effect, assist in engraftment, and protect against infections; however, they are also involved in the development of graft-versus-host-disease (GVHD). Although total nucleated cell (TNC) dose and CD34 cell doses have previously been correlated with post-transplant outcomes, there are limited data on the role of donor T-cell subsets in engraftment and post-HCT complications including GVHD. We examined the association of donor cell subset data with outcomes in myeloablative allogeneic HCT recipients from 2002 to 2014 in our single-center retrospective cohort study.
Analysis was restricted to recipients of BM (N=359) or G-CSF mobilized PBSC (N=61) grafts from HLA-identical sibling and unrelated donors. Total nucleated cell (TNC), CD34, CD3, CD4, CD8, and CD56 (PBSC only) doses from the pre-infusion graft were analyzed for association with disease and outcome data prospectively collected by our institution's HCT database. Multivariable Fine and Gray or Cox regression analyses adjusted for clinical variables and evaluated association of donor cell subset with the following outcomes: neutrophil engraftment (>500/uL x 3 days), platelet engraftment (>20,000/uL x 3 days), lymphocyte recovery (>500/uL x 1 day), any infection, donor chimerism (≥95% by day 100), grade 2-4 acute GVHD, chronic GVHD, non-relapse mortality (NRM) and overall survival. Analyses were performed separately for BM and PBSC groups.
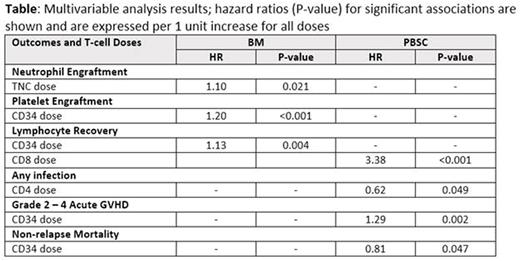
In the BM cohort, median recipient age was 47 years; 77% patients had acute leukemia/MDS; 58% had HLA-identical sibling donor. Most patients received ablative Bu-Cy (63%) or etoposide-TBI (19%) conditioning, with CNI-MMF (49%) or CNI-MTX (47%) as GVHD prophylaxis. Donor median age was 42 years and 39% were CMV positive. The mean graft (/kg) TNC and CD34 doses were 2.29x108 and 2.52x106, and mean T-cell subset doses were 32.19x108 CD3, 17.92x108 CD4, and 12.75x108 CD8, respectively. Among patients receiving a PBSC graft, median age was 48 years, 82% had acute leukemia/MDS, and 64% had an unrelated donor. Similar to BM, most patients received ablative Bu-Cy (67%) or etoposide-TBI (15%) conditioning, and CNI-MMF (33%) or CNI-MTX (61%) for GVHD prophylaxis. Median donor age was 40 years and 36% were CMV seropositive. The mean graft cell doses (/kg) were 9.51x108 TNC, 5.50x106 CD34, 2.74x108 CD3, 1.75x108 CD4, 0.88x108 CD8, and 0.36x108 CD56, respectively. Median time to neutrophil, lymphocyte and platelet recovery was 15, 27 and 23 days, respectively in the BM cohort, and 12, 19 and 16 days, respectively in the PBSC cohort. The table shows significant associations between donor cell subsets and post-HCT outcomes from the multivariable analysis.
In summary, among myeloablative allogeneic HCT patients receiving BM grafts, earlier neutrophil engraftment was seen with higher TNC dose, while platelet engraftment and lymphocyte recovery was seen with higher CD34 dose. Among PBSC grafts, higher CD8 dose was associated with earlier lymphocyte recovery, higher CD4 dose with lower risk of infection, and higher CD34 dose with greater risk of acute GVHD but lower risk of NRM. The relative contribution of each donor cell subset between BM and PBSC grafts can impact outcomes. We did not observe any association of donor cell subsets with donor chimerism, chronic GVHD, or overall survival in this population. BM and PBSC graft composition can impact myeloablative allogeneic HCT outcomes and future studies to evaluate optimal graft composition are needed.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.