Abstract
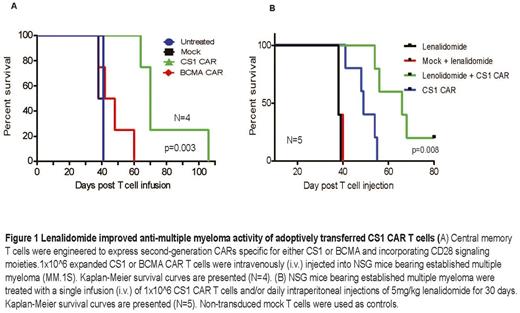
Multiple myeloma (MM) is an incurable malignancy of plasma cells even with great advances in treatment. Chimeric Antigen Receptor (CAR) directed T cell therapy, which can specifically recognize tumor associated antigens and kill tumor cells in an MHC independent manner, is a promising approach for hematological malignancy. There are several candidate antigens for CAR T cell targeting of multiple myeloma, including BCMA and CS1. Our goal is to develop novel CARs for the treatment of MM and explore the potential benefits of combinatorial therapy of CAR T cells and immunomodulatory drugs (IMiDs) such as lenalidomide. In the present study, we redirected central memory T cells to express second-generation CARs specific for either CS1 or BCMA that incorporate CD28 signaling moieties. Central memory T cells were activated by CD3/CD28 bead stimulation, transduced with lentivirus encoding the CAR construct, and expanded ex vivo. The engineered and expanded CS1 and BCMA CAR T cells exhibited similar phenotypes and comparable in vitro effector function. However, once adoptively transferred into MM tumor-bearing NOD/Scid IL2RγCnull (NSG) mice by intravenous injection of 1x10^6 CAR T cells, CS1 CAR T cells exhibited superior antitumor activity over BCMA CART cells and significantly prolonged mouse survival (P<0.01). To further improve the anti-MM activity of CAR T cell therapy, we investigated the effects of lenalidomide on CS1 CAR T cell function against MM. Central memory T cells were activated and transduced with lentivirus encoding CS1 CAR and then expanded in vitro in the presence of 0, 1 or 10mM lenalidomide for 3-4 weeks and then effector function was evaluated. We found that CD8+ CAR T cells were preferentially expanded over CD4+ CAR T cells in a dose-dependent manner. Lenalidomide-treated CAR T cells secreted higher levels of Th1 cytokines such as IFN-gamma, TNF-alpha, and IL-2, but reduced Th2 cytokines such as IL-4 and IL-10 upon antigen stimulation as compared with untreated CAR T cells. Meanwhile we observed that lenalidomide greatly improved the maintenance of T cell memory markers (CD62L, CD28, and CD27) in the culture and enhanced the formation of immune synapses between CAR T cells and MM cells. RNA-seq analysis revealed that more than 600 genes were differentially expressed among the lenalidomide treated and un-treated CD8+CAR+ T cells. Among those, expression of immune synapse related genes such as cell junction and biological assembly is significantly increased with lenalidomide treatment. Moreover, lenalidomide results in elevated gene transcrips characteristic of memory, homing and cytolytic function of CAR T cells. To test the synergistic effects, MM bearing mice were treated with a single infusion of 1x10^6 CS1 CAR T cells (i.v) on day 0 and/or 5-7.5mgkg-1 of lenalidomide daily (i.p.) initiating on day 0 for 30 days. CS1 CAR T cells and lenalidomide exhibited synergistic anti-MM activity in vivo when MM mice received combinatorial treatment. The combination therapy significantly inhibited tumor growth in vivo, prolonged mouse survival (P<0.01) and improved CAR T cell persistence in mice as compared to single-agent treatment. Taken together, these findings indicate that lenalidomide plays a co-stimulatory role in immune modulation of CAR T cells and strengthens the anti-tumor activity of CS1 CAR T cells in vivo. Rational combination of these immunotherapeutic regimens is an effective strategy and the planned clinical trial will use a combination of lenalidomide and CS1 CAR T cells for increasing treatment efficacy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.