Abstract
Chimeric antigen receptor T cells (CAR T) re-directed to CD19, have induced remarkable responses in clinical trials for patients with B-cell malignancies. Patients have responded to therapy with a CAR T dose which is a fraction of the pre-existing tumor burden. Explanations for this observation include studies which show the proliferative potential of the CAR T cells (Kalos M et al Sci Transl Med 2011), as well as our recent study which showed that individual CAR T cells can serial kill tumor cells (Davenport AJ et al CIR 2015, Figure 6). Using our dual antigen receptor model (OT-I T cell receptor and 2nd generation HER-2 CAR in the same T cell, termed CAR.OTI cells), we also observed a reproducible and significantly shorter time from CAR- vs TCR-mediated activation to detachment from dying tumor cells (Davenport AJ et al CIR 2015, Figure 4D)suggesting that CAR-mediated individual killing events are actually faster. To explore how this may occur, we examined the immune synapse structure at 20 minutes of CAR.OTI CTL co-culture with tumor cells expressing the cognate antigen for either the CAR or TCR. At this timepoint, CAR.OTI CTL, activated via the TCR, formed a conventional bull's eye immune synapse with accumulation of LCK and actin clearance (Figure 1). Surprisingly, CAR.OTI CTL activated via their CAR had an immune synapse with no or diffuse LCK and small actin rings (Figure 1). At the same timepoint, CAR-activated CAR.OTI CTL conjugates with tumor cells were characterized by a microtubule organizing center (MTOC) distant from the immune synapse LCK accumulation. In contrast TCR activated CAR.OTI CTL conjugates consistently had the MTOC proximal to the LCK accumulation (Figure 2A). Despite this, CAR-mediated CAR.OTI CTL killing of tumor targets was inhibited by a protein kinase C zeta inhibitor and is, therefore, MTOC dependent (Figure 2B). The MTOC circumnavigates the activated CTL nucleus and moves to the immune synapse, bringing cytotoxic granules with it. Using time lapse live video (TLLV) microscopy, we compared CAR.OTI CTL cytotoxic granule movement when the CAR.OTI were activated via the TCR vs CAR. We showed that following CAR vs TCR activation, CAR.OTI cytotoxic granules moved with a significantly higher velocity, and had a shorter time lapse to reach the immune synapse following activation (Ca2+ flux), and a significantly shorter time to detachment from the dying tumor cell (Figure 2C).
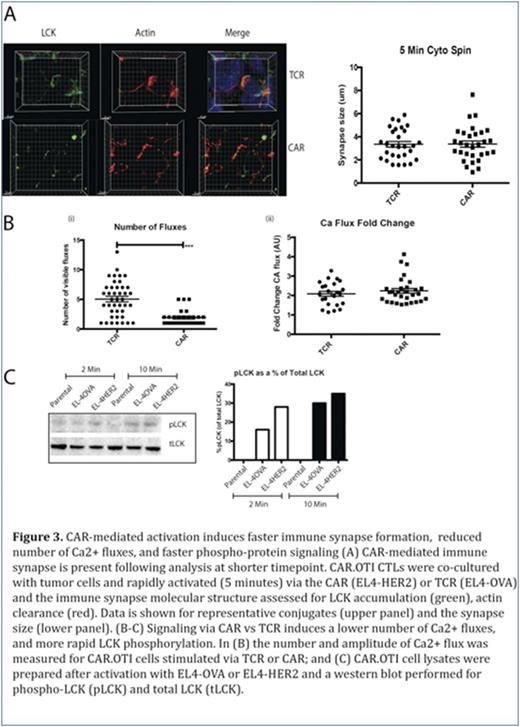
We then re-examined immune synapse formation at an earlier timepoint, to explore whether the data from Figures 1-2 could be explained by a more rapid CTL response following CAR-mediated signaling. In contrast to our observations at 20 minutes, at five minutes we showed CAR-activated CAR.OTI CTL formed conjugates with tumor targets and the immune synapse showed distinct LCK accumulation and actin clearance (Figure 3A). Finally, we explored CAR.OTI CTL signaling and showed that CAR-mediated activation induced a significantly lower number of Ca2+ fluxes, however each Ca2+ flux amplitude was not different (Figure 3B). We also examined changes in proximal (phospho-LCK, pLCK) and distal (phospho-ERK, pERK) signals in CAR- versus TCR- activated CAR.OTI cells, and showed that CAR-mediated activation induced more rapid proximal and distal activation signals (Figure 3C). In conclusion, this study showed that compared to activation by TCR ligation, T cells respond to CAR ligation with faster phospho-protein signaling, Ca2+ flux, formation of an immune synapse and a more rapid movement of the MTOC and delivery of the cytotoxic granules to kill the tumor cells. Furthermore, LFA-1 did not accumulate at the immune synapse following CAR activation, therefore, reduced adhesion may facilitate the observed rapid detachment from the dying tumor cell, and enable the CAR T to rapidly move onto the next tumor target for 'bigger, stronger, faster' killing.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.