Abstract
Introduction: Yes-associated protein (YAP), a direct downstream effector of the tumor suppressive Hippo signaling, functions as a transcriptional coactivator in the modulation of cell growth, proliferation and apoptosis in a number of cancers. Given that Hippo signaling does not have an extracellular ligand nor a specific membrane receptor, its activation depends on the crosstalk mechanisms. Receptor tyrosine kinase (RTK) insulin-like growth factor-1 receptor (IGF-1R) is implicated in various tumor entities by modulating the proliferation, survival and metastasis of cancer cells. Strategies to block IGF-1R pathway in solid malignancies are being tested in clinical trials. However, the potential significance of IGF-1R and YAP in DLBCL remains ill defined. We assume that IGF-1R modulates the proliferation and apoptosis of DLBCL cells partly by regulating YAP in DLBCL. We aimed to investigate the functional significance of IGF-1R and its regulatory effect on YAP in DLBCL.
Methods: Lymph node biopsies from 30 de novo DLBCL patients and 20 reactive hyperplasia cases were collected with informed consents. Immunohistochemistry (IHC) was performed to assess the expression of YAP and IGF-1R in lymphoma tissues. Protein expression levels of YAP and IGF-1R in DLBCL cell lines were detected by western blotting. Peripheral blood mononuclear cells were isolated with informed consents from healthy volunteers. Effects of AG1024 on cell viabilities and apoptosis were assessed by cell counting kit-8 and Annexin-V/7-AAD staining. Propidium iodide staining was conducted to assess cell cycle of DLBCL cells.
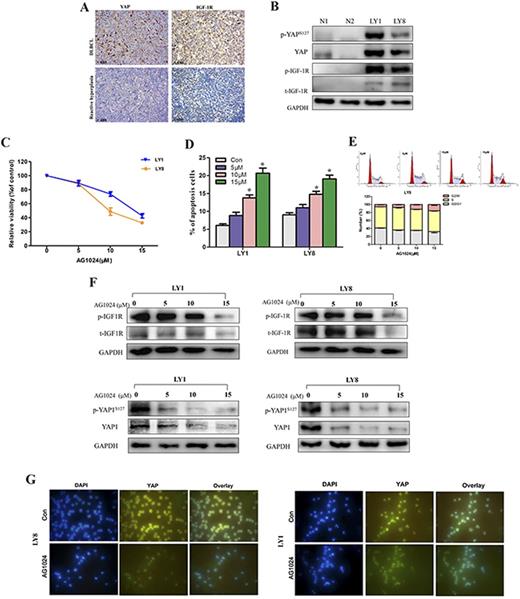
Results: Compared to the reactive hyperplasia group, DLBCL patients revealed significantly higher protein levels of both YAP and IGF-1R (Fig. 1A). Overexpression of YAP and IGF-1R protein in DLBCL cell lines (LY1 and LY8) were further confirmed by immunoblotting (Fig. 1B). AG1024, a selective inhibitor of IGF-1R, significantly restrained the viabilities of LY1 and LY8 cells in a dose dependent manner (Fig. 1C). Apoptotic rates in LY1 and LY8 cells treated with IGF-1R inhibitor were markedly increased (Fig. 1D). Moreover, addition of IGF-1Rsuppressor induced remarkable cell cycle arrest in G2/M phase in DLBCL cells (Fig. 1E). In LY1 and LY8 cells treated with AG1024, we observed that the reduced phosphorylation of IGF-1R was accompanied by significantly declined level of YAP protein level (Fig. 1F). Results of the immunofluorescense verified the inhibitory effect of AG1024 on expression of YAP protein in LY1 and LY8 cell (Fig. 1G).
Conclusions: We identified the aberrant overexpression of YAP and IGF-1R in DLBCL patients and cell lines, which suggesting the dysregulation of Hippo and IGF-1 signaling in DLBCL. IGF-1R inhibitor exhibits proliferation-inhibitory and pro-apoptotic effect via inhibiting the activation YAP. We provided a novel mechanism involved in the function of IGF-1R in DLBCL. Further interrogation on the biological function of YAP in DLBCL will highlight the crosstalk between these two pathways and presents a promising therapeutic strategy in DLBCL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.