Abstract
Background: Though CD19 is expressed only rarely on multiple myeloma (MM) plasma cells (PC), rare CD19+ B cells can be identified in MM patients that are clonally related to the MM PC. These clonotypic B cells may exhibit properties of cancer stem cells (enhanced MM-propagating properties and drug resistance compared to MM PC) and thus be a potential therapeutic target in conjunction with therapies that target MM PC. CTL019 consists of autologous T cells transduced via lentiviral vector with an anti-CD19 scFv coupled to CD3-zeta and 4-1BB signaling domains and expanded ex vivo with anti-CD3/CD28-conjugated beads. To target both clonotypic B cells and MM PC, we conducted a pilot clinical trial of CTL019 administered after high-dose melphalan and autologous stem cell transplantation (ASCT) in relapsed/refractory MM patients who had previously undergone first-line ASCT with short progression-free survival (PFS).
Methods: Subjects were required to be medically fit for ASCT and have progressed within 1 year of a prior ASCT performed as part of first-line therapy. Study therapy consisted of ASCT with melphalan 140-200 mg/m2 followed by 1-5x107 CTL019 cells 12-14 days later. The primary endpoint was safety and feasibility of CTL019 manufacturing and administration in this clinical setting. Secondary endpoints included assessments of CTL019 in vivo persistence and activity against normal B cells, plasma cell immunophenotype as a response biomarker, and PFS after ASCT + CTL019 in comparison to PFS after initial ASCT.
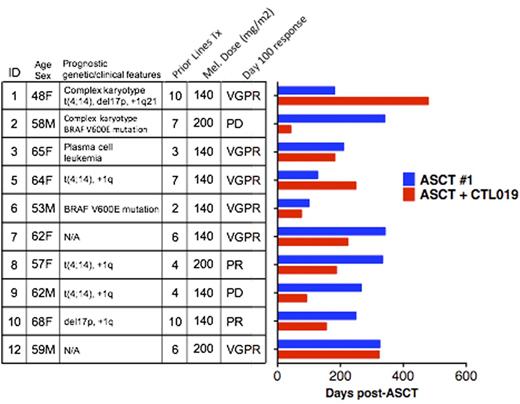
Results: Twelve subjects enrolled, and 10 received study therapy; autologous T cells failed to expand ex vivo in one enrolled subject, and one enrolled subject elected to pursue off-study therapy. Median age was 61 (range 48-68). Median prior lines of therapy was 6 (range 2-10). Poor-prognosis features were present in 8/10 subjects (6/10 with poor-prognosis cytogenetics, 2/10 with BRAF V600E mutations, 1/10 with secondary plasma cell leukemia). Median PFS after first-line ASCT was 258 days (range 100-342). In pre-ASCT bone marrow (BM), the dominant MM PC population was CD19-negative by flow cytometry in 9/9 evaluable subjects, though 7/9 exhibited rare CD19+ subsets comprising 0.05-1.5% of MM PC. Melphalan dose was 140 (N=7) or 200 (N=3) mg/m2. All subjects infused received the maximum target dose of 5x107 CTL019 cells. Adverse events (AE) consisted mostly of expected ASCT toxicities. Grade ³3 AE that were at least possibly related to CTL019 included grade 3 autologous GVHD (N=1, resolved with corticosteroids) and oral mucositis (N=1). Grade 1 cytokine release syndrome occurred in 1 subject. There was no ASCT-related mortality. After infusion, CTL019 cells were detectable in peripheral blood (PB) of all subjects and persisted for median of 44 days (range 14-156). Presence of PB CTL019 cells was associated with absence of PB B cells. Notably, CTL019 cells were detected in BM in 9/10 subjects at day 42 and/or 100 post-ASCT. Median PFS after ASCT + CTL019 was 185 days (range 42-479); all subjects have progressed. The peak BM CTL019 frequency correlated significantly with favorable PFS (SpearmanÕs rho=0.77, P=0.009). There was no association between PFS and peak frequency of CTL019 or duration of CTL019 persistence in PB. In 3/10 subjects, PFS after ASCT + CTL019 met or exceeded PFS after first-line ASCT (Figure). For comparison, in a historical cohort of 18 patients who received first-line and salvage ASCT at our institution since 2008, no patients exhibited longer PFS after salvage ASCT.
Conclusion: CTL019 manufacturing and administration post-ASCT is safe and feasible in patients with advanced MM. Correlation of PFS with CTL019 frequency in BM and prolonged PFS in 3 subjects is suggestive of clinical efficacy. A phase-two study of CTL019 using a 10-fold higher dose after first-line ASCT in high-risk MM patients is ongoing.
Garfall:Medimmune: Consultancy; Bioinvent: Research Funding; Novartis: Consultancy, Research Funding. Stadtmauer:Novartis: Consultancy; Takada: Consultancy; Janssen: Consultancy; Amgen: Consultancy; Teva: Consultancy; Celgene: Consultancy. Maus:Novartis: Patents & Royalties: related to CTL019, Research Funding. Hwang:Novartis: Research Funding. Vogl:Takeda: Consultancy, Research Funding; GSK: Research Funding; Calithera: Research Funding; Teva: Consultancy; Constellation: Research Funding; Celgene: Consultancy; Acetylon: Research Funding; Karyopharm: Consultancy. Cohen:Bristol-Meyers Squibb: Consultancy, Research Funding; Janssen: Consultancy. Weiss:Novartis: Consultancy. Porter:Genentech: Employment; Novartis: Patents & Royalties, Research Funding. Frey:Novartis: Research Funding; Amgen: Consultancy. Milone:Novartis: Patents & Royalties, Research Funding. Mangan:Novartis: Speakers Bureau. Lacey:Novartis: Research Funding. Melenhorst:Novartis: Patents & Royalties: Novartis, Research Funding. Ambrose:Novartis: Research Funding. Chen:Novartis: Research Funding. Kulikovskaya:Novartis: Research Funding. Levine:Novartis: Patents & Royalties, Research Funding; GE Healthcare Bio-Sciences: Consultancy. June:Johnson & Johnson: Honoraria; Tmunity Therapeutics: Equity Ownership; Novartis: Honoraria, Patents & Royalties, Research Funding; Immune Design: Consultancy, Equity Ownership; Celldex: Consultancy, Equity Ownership; Novartis: Honoraria, Patents & Royalties, Research Funding; Pfizer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.