Abstract
Background. Autologous stem cell transplantation (ASCT) remains a gold standard treatment for younger patients with multiple myeloma (MM). With the aid of new drugs, the patients live longer and third salvage ASCT is an increasingly used option. However, the outcome of third salvage ASCT has not yet been analyzed.
Methods. Between 1997 and 2010, 1288 MM patients who had received at least 3 ASCT were registered in the EBMT database. We excluded patients older than 75 years, those with a time from diagnosis to first ASCT of more than 10 years, patients transplanted with bone marrow, those with a time between relapse and ASCT of less than one month and those with an uncertain relapse date. Planned tandem ASCT was considered to be performed within a 6 month interval and at least 1 month apart. The conditioning regimen for the third transplant was high dosemelphalan alone or in combination withbusulfan orbortezomib. We could distinguish two main groups: 417 patients who received tandem ASCT and then a third ASCT after single relapse (AARA group) and 72 patients who received one ASCT, a second ASCT after first relapse, and a third ASCT after second relapse (ARARA group). A third group, who received tandem ASCT after relapsing following single ASCT comprised only 25 patients and was not studied.
Results. We compared the two groups AARA vs ARARA. A third ASCT was performed in the AARA group in more recent years (p=0.047). There were more males than females (65% vs 72%) in both groups (p=NS). The main myeloma isotype was IgG (56% vs 58%). The ISS stage at diagnosis was similar (stage III in 70% vs 72% of cases). The time interval between diagnosis and first ASCT tended to be shorter in the AARA group (p=0.089), being within the first 6 months in 50% vs 38% of patients. The status at third ASCT was different: CR, 5% vs 4%: VGPR or PR, 45% vs 19%: MR or stable disease, 12% vs 15%: primary refractory/progressive disease, 38% vs 62% (p<0.001). A Karnofsky score of >70% at third ASCT was reported in 91% vs 90% of cases. The conditioning regimen at third ASCT was different between the two groups: melphalan 200 mg/m2, 42% vs 18%: melphalan 140 mg/m2, 6%vs 12%: other 52%vs 70% (p=0.018). The median age at third ASCT was 59vs 61 years. There was a trend to a longer time between first ASCT and first relapse (27vs 22 months (p=0.056)) in the AARA group while the interval between first ASCT and third ASCT was much shorter (44vs 64 months (p<0.001)). The time between the last relapse and third ASCT was similar (9vs 11 months (p=0.4)).
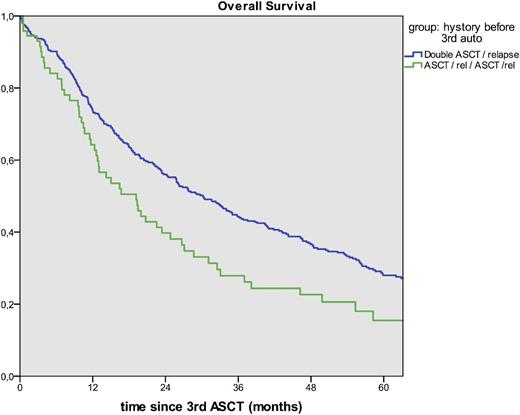
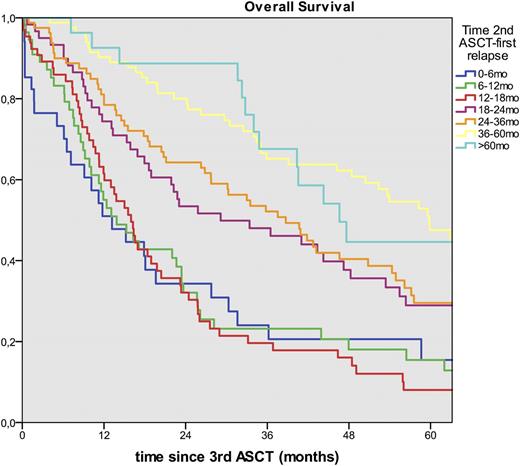
The median follow-up was similar (70vs73 months (p=0.9)). Engraftment was similar (96%vs93% (p=0.35)).The best response achieved after the third ASCT was superior in the AARA group: CR, 32%vs12%: VGPR or PR, 60%vs71% :MRor SD, 4%vs14%: progression, 4%vs3% (p<0.001). The median OS after third ASCT was much higher in the AARA group: 30vs19 months (p=0.01) (Figure 1). The causes of death were: relapse/progression, 84%vs84%: second primary malignancies (SPM), 0.4%vs3.6%: other, 16%vs13%. There was a trend to more SPM including both hematologic and solid tumors in the ARARA group (p=0.068) with a shorter median time to SPM, 12vs42 months (p=0.004). Focusing on the AARA group, the longer the time from tandem ASCT to first relapse, the better the OS after the third transplant: if relapse occurred within 12 months of tandem ASCT, median OS of 13 months: within 18-24 months, OS of 29 months: within 36-60 months, OS of 59 months (Figure 2).
Conclusions.A third ASCT is feasible in MM with more than 80% of patients achieving at least PR although with increased non relapse mortality. This treatment is mostly used in one of two scenarios: tandem ASCT followed by relapse and a third ASCT, or less commonly a first ASCT followed by a first relapse, a second ASCT, a second relapse and subsequently a third ASCT. The first scenario gives much better results, partly due to a better remission status at the third ASCT with no sign of increased SPM. In this AARA group, if relapse occurred more than 3 years after the initial tandem ASCT, the median OS after third ASCT was more than 4 years. These results should be compared with those obtained with the new drugs, especially in combination.
AARA group: Overall Survival after the third transplant according to the time to relapse following tandem ASCT.
AARA group: Overall Survival after the third transplant according to the time to relapse following tandem ASCT.
Garderet:Takeda: Consultancy; Amgen: Consultancy; BMS: Consultancy, Honoraria; Novartis: Consultancy. Dreger:Janssen: Consultancy; Gilead: Speakers Bureau; Novartis: Speakers Bureau; Novartis: Consultancy; Gilead: Consultancy; Roche: Consultancy. Leleu:TEVA: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; LeoPharma: Honoraria; Pierre Fabre: Honoraria; Amgen: Honoraria; Bristol-Myers Squibb: Honoraria; Takeda: Honoraria; Celgene: Honoraria; Janssen: Honoraria. Peschel:MophoSys: Honoraria. Meuleman:Bristol-Myers-Squibb: Consultancy; Celgene: Consultancy; Amgen: Consultancy; Takeda: Consultancy. Ciceri:MolMed SpA: Consultancy. Schönland:Janssen, Prothena, GSK: Consultancy, Employment, Equity Ownership, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommations, Patents & Royalties, Research Funding, Speakers Bureau. Kröger:Sanofi: Honoraria, Research Funding; Neovii: Honoraria, Research Funding; Riemser: Honoraria, Research Funding; Novartis: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract