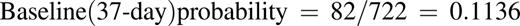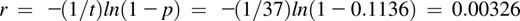Key Points
Fondaparinux was the most cost-effective agent for suspected HIT, prevailing over both argatroban and bivalirudin.
Our data suggest potential cost savings with fondaparinux and underscore the need for larger clinical studies of fondaparinux in this area.
Despite the availability of multiple nonheparin anticoagulants for the treatment of heparin-induced thrombocytopenia (HIT), few data are available comparing the cost-effectiveness of these agents. This analysis is particularly important when considering differences in the risk of adverse effects, routes of administration, requirements for phlebotomy and laboratory monitoring, and overall drug costs. We conducted a cost-effectiveness analysis of argatroban, bivalirudin, and fondaparinux for the treatment of suspected HIT from the institutional perspective. A 3-arm decision-tree model was developed that employs standard practices for anticoagulation monitoring. We incorporated published data on drug efficacy and probability of HIT-related thromboembolism and major bleeding. We considered both institutional costs and average wholesale price (AWP) and performed probabilistic sensitivity analyses (PSA) to address any uncertainty in model parameters. Using institutional costs, fondaparinux prevailed over both argatroban and bivalirudin in terms of cost ($151 vs $1250 and $1466, respectively) and adverse events averted (0.9989 vs 0.9957 and 0.9947, respectively). Results were consistent when AWP was used, with fondaparinux being less expensive ($555 vs $3081 and $2187, respectively) and more effective in terms of adverse events averted (0.9989 vs 0.9957 and 0.9947, respectively). The PSA confirmed our findings using both institutional costs and AWP. In conclusion, fondaparinux subcutaneous injection afforded significant advantages in terms of cost savings and adverse events averted compared with IV argatroban or bivalirudin infusions. Our data strongly suggest potential cost savings with fondaparinux and underscore the critical need for larger clinical studies of fondaparinux in the treatment of suspected HIT.
Introduction
Heparin-induced thrombocytopenia (HIT) is an uncommon yet serious adverse effect of heparin products. This complication develops due to the formation of an immunogenic complex of negatively charged heparin with the positively charged platelet factor 4 that forms on the surface of activated platelets. Immunoglobulin G antibodies form and bind to the platelet factor 4–heparin complex, ultimately leading to the activation of the coagulation cascade with potential arterial and venous thrombotic complications.1,-3 HIT is the most frequent drug-induced type of thrombocytopenia, with an incidence of ∼1% to 5% of inpatients exposed to heparin in the United States.2,4 Management of strongly suspected or confirmed HIT includes cessation of heparin products and initiation of alternative anticoagulant therapy to inhibit thrombin production while awaiting laboratory testing and clinical evaluation for thrombosis to confirm or rule out the diagnosis of HIT.2
Treatment of suspected and confirmed HIT has traditionally involved the use of nonheparin anticoagulants, including direct thrombin inhibitors (argatroban, bivalirudin, and lepirudin) or heparinoids (danaparoid). Argatroban and bivalirudin are the currently available US Food and Drug Administration (FDA)–approved anticoagulants for the management of HIT,5,6 although bivalirudin’s approved indication is specifically for treatment of HIT in patients undergoing percutaneous coronary intervention. The evidence supporting the management of suspected HIT patients with bivalirudin stems mainly from case reports.7,,,,,-13 These agents are expensive, administered intravenously as continuous titratable infusions, and require frequent laboratory monitoring and dose adjustment.
Fondaparinux has received increased attention as a nonheparin anticoagulant in the treatment of suspected HIT.14,,-17 Expert opinion3,18,-20 and emerging evidence21,-23 suggest fondaparinux is a safe and effective alternative agent. This selective factor Xa inhibitor is provided as a daily subcutaneous injection and requires only infrequent laboratory monitoring. Considering its potential clinical utility, the relatively low drug cost, and the lack of frequent laboratory monitoring and dose titration, fondaparinux may provide a reduced risk of adverse effects as well as significant cost savings over FDA-approved agents. We performed a cost-effectiveness analysis comparing argatroban, bivalirudin, and fondaparinux in the management of suspected HIT from the institutional perspective. In this, we focused on the costs associated with managing the adverse events of venous thromboembolism (VTE) and major bleeding, laboratory tests, drug administration time, and drug costs to derive conservative estimates of the comparative cost-effectiveness of these agents in the management of suspected HIT.
Methods
Patient population
The modeled cohort used the profile of patients in relevant trials: adult men and nonpregnant, nonbreastfeeding women with creatinine clearance >50 mL/min and suspected or confirmed HIT.24,25 A diagnosis of HIT was assumed to include a 4T score >4 with a positive enzyme-linked immunosorbent assay (ELISA) optical density >0.4 and a confirmed platelet serotonin-release assay (SRA).2,26
Model structure
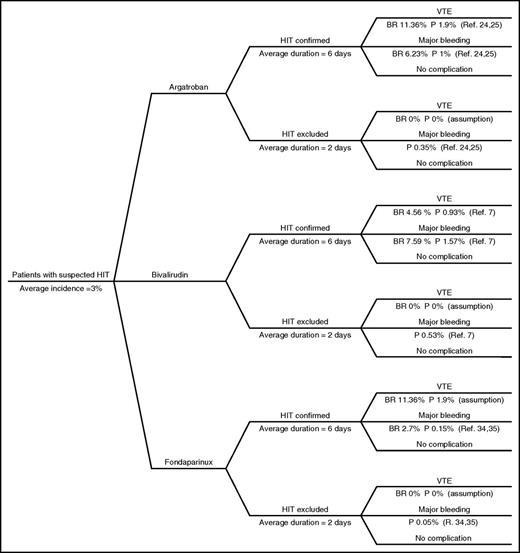
A decision-tree model was developed to simulate a hypothetical cohort of patients with suspected HIT treated with argatroban, bivalirudin, or fondaparinux as alternative anticoagulants to the discontinued heparin products (Figure 1). This model was designed to calculate cost in US dollars (USD) per adverse event averted. The model was tested in probabilistic sensitivity analyses (PSA) with 2000 simulations using specifically programmed routines in Excel (Microsoft Corporation, Redmond, WA) that we have used extensively in prior economic analyses.27 The model specifies 3 pathways of clinical decision making once HIT is suspected. Heparin products are discontinued and management with argatroban, bivalirudin, or fondaparinux is initiated. Laboratory diagnostic tests to confirm or rule out HIT are ordered. While awaiting the results, patients are at risk of VTE or major bleeding. We used 3% as the mean incidence of confirmed HIT based on the reported incidence of 1% to 5% in inpatients in the United States.2,4 Although higher percentages of up to 10% have been reported in different subpopulations of patients,28,-30 we opted for an incidence rate reflecting the general population.
Decision-analysis tree. The decision-analysis tree model starts when HIT is suspected and all forms of heparin are discontinued. Hypothetical patients are initiated on argatroban, bivalirudin or fondaparinux. The model assumes laboratory tests would be readily available after 2 days to confirm HIT diagnosis and continue treating patients with nonheparin anticoagulants for a total of 6 days or exclude the diagnosis of HIT and stop nonheparin anticoagulant after 2 days. The model assumed 3 expected outcomes while on nonheparin anticoagulant: HIT-related VTE, major bleeding, or no complication. The baseline rates of outcomes reported in studies and the calculated probabilities of these outcomes can be seen in this figure. BR, baseline rate; P, probability; Ref, reference.
Decision-analysis tree. The decision-analysis tree model starts when HIT is suspected and all forms of heparin are discontinued. Hypothetical patients are initiated on argatroban, bivalirudin or fondaparinux. The model assumes laboratory tests would be readily available after 2 days to confirm HIT diagnosis and continue treating patients with nonheparin anticoagulants for a total of 6 days or exclude the diagnosis of HIT and stop nonheparin anticoagulant after 2 days. The model assumed 3 expected outcomes while on nonheparin anticoagulant: HIT-related VTE, major bleeding, or no complication. The baseline rates of outcomes reported in studies and the calculated probabilities of these outcomes can be seen in this figure. BR, baseline rate; P, probability; Ref, reference.
The 3 pathways split into secondary pathways based on diagnostic test results (ELISA and/or SRA) confirming or ruling out HIT. Since it usually takes ≥24 hours for these results,31 it was assumed that patients in whom HIT was ruled out were exposed to 2 days of nonheparin anticoagulation. In patients with confirmed HIT, we assumed a best-case scenario of platelet recovery to >150 × 109/L and bridging to warfarin requiring 4 additional days of nonheparin treatment, for a total of 6 days.3,19,24,25
The 6 pathways are associated with specific probabilities of developing HIT-related VTE or experiencing an HIT-related major bleeding based on published incidence rates (Figure 1). The major bleeding observed in relevant trials was gastrointestinal (GI) bleeding.5,32 Thus, we assumed that the cost of managing GI bleeding equated the cost of managing major bleeding. We assumed a zero probability of HIT-related VTE in HIT-negative patients. Amputation was not included as an adverse event due to insufficient published evidence regarding amputation rates associated with fondaparinux therapy; which would disfavor argatroban.33 Death was not included as an adverse event; relevant deaths in the argatroban trials were those attributable to HIT-related VTE or major bleeding and therefore events would be duplicated in our analysis.24,25 Based on published evidence, the daily probability of HIT-related VTE associated with fondaparinux was assumed to be the same as that associated with argatroban.17 However, each agent has a different probability of major bleeding.24,25,34,35
Probabilities for major bleeding were converted, as needed, to 2 and 6 days as follows: p = 1 − exp (−rt), where p is probability, r is rate, and t is time.
For example, the following calculates the 6-day probability of HIT-related VTE associated with argatroban that was measured over 37 days in the pivotal trials24,25 :
This estimate assumes that the daily rate of adverse events is constant over time.
Cost estimates
All costs were expressed in 2016 USD; those not expressed in 2016 USD were adjusted using the consumer price index.36 The argatroban package insert recommends 2 μg/kg per minute for patients without liver dysfunction,5 although in certain patient populations, starting with lower doses may achieve therapeutic activated partial thromboplastin time (aPTT) and improve drug safety.37,-39 We used a conservative, low dose of 0.5 μg/kg per minute. The off-label dose of bivalirudin for managing HIT is suggested as 0.15 mg/kg per hour as a continuous titratable infusion.14 Due to uncertainty with regard to therapeutic vs prophylactic fondaparinux dose for management of suspected HIT,40 we used therapeutic fondaparinux dosing per the American College of Chest Physicians guideline on fondaparinux for suspected HIT.2 Considering an average body weight for North American adults of 80.7 kg,41 we assumed a total daily dose of 58.1 mg argatroban, 291.5 mg bivalirudin, and 7.5 mg fondaparinux. Argatroban and bivalirudin are only stable for 24 hours after preparation.5,6 This means there would be significant waste of argatroban, using only 58.2 mg from each 250-mg vial, and minimal waste of bivalirudin. Therefore, our analysis included use of 6 vials for those receiving argatroban and 7 vials for those receiving bivalirudin for a total of 6 days of therapy. The argatroban package insert recommends performing 1 aPTT test 2 hours after initiation and after each dose adjustment.5 Bivalirudin is also titrated for HIT therapy based on trends in the aPTT. To obtain the most conservative estimate of argatroban and bivalirudin cost, we assumed that patients would achieve a therapeutic aPTT with 0.5 μg/kg per minute dose for argatroban and 0.15 mg/kg per hour for bivalirudin, precluding the need for dose adjustment and repeat test. Monitoring of anti–factor Xa with fondaparinux is not routinely recommended and was not included in our analysis.
We used the drug cost at our institution (Banner-University Medical Center, Tucson, AZ) as primary base-case estimate: a vial cost of $331 for 250 mg/2.5 mL argatroban, $418 for a 250-mg/5-mL vial of bivalirudin, and a prefilled 7.5-mg fondaparinux syringe cost of $32.72. We performed a secondary base-case analysis utilizing the average wholesale price (AWP) from Red Book for national costs.42
Costs of adverse events and aPTT tests were estimated from published literature.43,-45 Administration costs were calculated per the 2016 Medicare Physician Fee Schedule using the Current Procedure Terminology codes (Table 1).46 We excluded costs that would be the same for the 3 agents (eg, the cost of ELISA and/or SRA; cost of tests such as serum creatinine, hemoglobin, hematocrit; and cost per day of hospitalization). These were assumed to be equal for all agents. More generally, we aimed to derive conservative estimates of the comparative cost-effectiveness of the 3 agents by focusing on a core set of costs so to favor, from the onset, argatroban and bivalirudin over fondaparinux.
Probabilistic sensitivity analyses
PSAs with 2000 simulations were performed on both the institutional costs and AWP to address the uncertainty in the model through sampling from distributions assigned to the model parameters. The uncertainty in treatment cost was addressed by applying γ distributions. We also applied γ distributions to the cost of managing adverse events. Uncertainty regarding the probability of adverse events was assessed using β distributions. One-way sensitivity analysis was implemented for argatroban and bivalirudin, and the vial cost in the primary base-case analysis was used to define the price at which the 2 agents would be equivalent to fondaparinux in terms of cost.
Additional sensitivity analyses
Longer duration of therapy.
To account for different scenarios encountered in clinical practice, we performed an additional analysis examining a longer (9-day) duration of nonheparin anticoagulants for the management of patients with confirmed HIT than the 6 days reported in pivotal trials. This 9-day duration accounts for a median of 4 days required for platelets to rise to >150 × 109/L and an additional 5 days of overlapping with warfarin to reach a therapeutic international normalized ratio (INR) goal as recommended by the American College of Chest Physicians guideline for the treatment of HIT.2 The additional analysis also assumed patients with confirmed HIT would require 9 vials of argatroban and 11 vials of bivalirudin over the 9 days. The model structure and assumptions otherwise remained the same as in the primary analysis utilizing the local costs estimates.
Lower incidence of HIT.
Because of variations in the incidence of HIT reported in different studies, we performed an additional sensitivity analysis incorporating a lower incidence of HIT, specifically the 0.2% rate reported by Smythe et al in 24 068 patients admitted to a large teaching hospital.47 The model structure and assumptions otherwise remained the same as in the primary analysis.
Variable adverse events rates.
One-way sensitivity analysis on the efficacy side was implemented for argatroban and bivalirudin. The baseline rate of adverse events for argatroban and bivalirudin were decreased to define the point at which argatroban and bivalirudin would dominate fondaparinux in terms of efficacy. The model structure and assumptions otherwise remained the same as in the primary analysis utilizing the local costs estimates.
Results
Analysis using institutional cost estimates
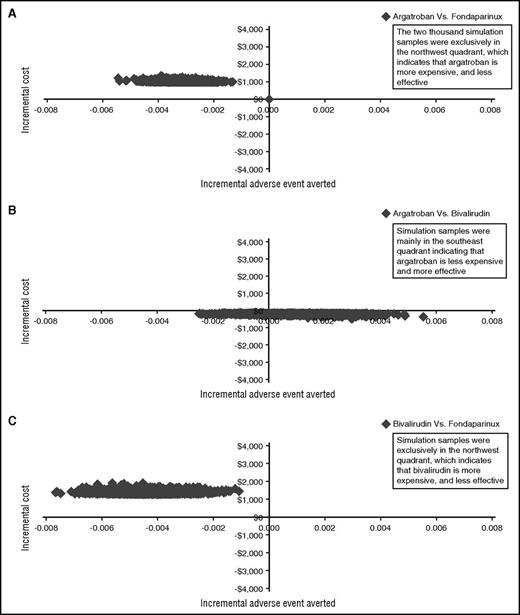
Using institutional cost estimates, the total costs for fondaparinux, argatroban, and bivalirudin treatment were $151, $1250, and $1466, respectively (Table 2). Fondaparinux was more effective than both argatroban and bivalirudin in terms of adverse events averted (0.9989 vs 0.9957 and 0.9947, respectively). Thus, fondaparinux prevailed over argatroban and bivalirudin, as it was less expensive in managing suspected HIT and more effective in terms of adverse events averted. Compared with bivalirudin, argatroban was less expensive and more effective in terms of adverse events averted. PSA results confirmed the base-case results, with fondaparinux being less expensive than both agents in managing suspected HIT and associated with better adverse events averted outcomes (Table 2; Figure 2A-C). One-way sensitivity analysis showed that the cost of argatroban and bivalirudin would never be lower than the cost of fondaparinux when the fondaparinux 7.5-mg syringe cost is set at $32.72. Even if the argatroban and bivalirudin prices declined to $10 while fondaparinux increased to $200, fondaparinux would remain cost-effective based on current evidence.
Probabilistic sensitivity analysis using institutional drug costs. Two thousand simulation samples were used to confirm analysis using institutional costs. y-axis represents the incremental cost and x-axis represents incremental adverse event averted. Samples in north quadrants indicate more expensive drug, while samples in south quadrants indicate more cost savings. East quadrants represent a higher rate of adverse events averted, and samples in west quadrants mean a lower rate of adverse events averted. (A) Argatroban compared with fondaparinux. (B) Argatroban compared with bivalirudin. (C) Bivalirudin compared with fondaparinux.
Probabilistic sensitivity analysis using institutional drug costs. Two thousand simulation samples were used to confirm analysis using institutional costs. y-axis represents the incremental cost and x-axis represents incremental adverse event averted. Samples in north quadrants indicate more expensive drug, while samples in south quadrants indicate more cost savings. East quadrants represent a higher rate of adverse events averted, and samples in west quadrants mean a lower rate of adverse events averted. (A) Argatroban compared with fondaparinux. (B) Argatroban compared with bivalirudin. (C) Bivalirudin compared with fondaparinux.
Analysis using AWP cost estimates
Secondary analysis with AWP data confirmed the primary analysis with local data that fondaparinux prevailed over argatroban and bivalirudin treatment, as it was less expensive in managing suspected HIT ($555 vs $3081 and $2187, respectively) and more effective in terms of adverse events averted (0.9989 vs 0.9957 and 0.9947, respectively) (Table 2). Argatroban was more expensive than bivalirudin but more effective in terms of adverse events averted. In PSA, fondaparinux was less expensive than argatroban and bivalirudin in managing suspected HIT and associated with better outcomes in terms of adverse events averted. Also, argatroban was more expensive but more effective in averting adverse events than bivalirudin (Table 2; Figure 3A-C).
Probabilistic sensitivity analysis using average wholesale prices. Two thousand simulation samples were used to confirm analysis using average wholesale prices. y-axis represents the incremental cost, and x-axis represents incremental adverse event averted. Samples in north quadrants indicate more expensive drug, while samples in south quadrants indicate more cost savings. East quadrants represent a higher rate of adverse events averted, and samples in west quadrants mean a lower rate of adverse events averted. (A) Argatroban compared with fondaparinux. (B) Argatroban compared with bivalirudin. (C) Bivalirudin compared with fondaparinux.
Probabilistic sensitivity analysis using average wholesale prices. Two thousand simulation samples were used to confirm analysis using average wholesale prices. y-axis represents the incremental cost, and x-axis represents incremental adverse event averted. Samples in north quadrants indicate more expensive drug, while samples in south quadrants indicate more cost savings. East quadrants represent a higher rate of adverse events averted, and samples in west quadrants mean a lower rate of adverse events averted. (A) Argatroban compared with fondaparinux. (B) Argatroban compared with bivalirudin. (C) Bivalirudin compared with fondaparinux.
Additional sensitivity analyses
Longer duration of therapy.
Applying institutional cost estimates, the total costs of fondaparinux, argatroban, and bivalirudin were $164, $1326, and $1562, respectively, assuming a 9-day treatment duration. Consistent with the primary analysis, fondaparinux was more effective in preventing adverse events compared with argatroban and bivalirudin (adverse events averted, 0.9986 vs 0.9953 and 0.9946, respectively). Employing AWP cost estimates also confirmed the results of the primary analysis, with fondaparinux prevailing over argatroban and bivalirudin in terms of cost savings ($579, $3235, and $2324) and adverse events averted (0.9986 vs 0.9953 and 0.9946), respectively.
Lower incidence of HIT.
The results of the analysis incorporating lower incidence of HIT confirmed the primary analysis results with fondaparinux prevailing over both agents. The total costs utilizing local cost estimates were $125 for fondaparinux, $1143 for argatroban, and $1343 for bivalirudin, with fondaparinux also prevailing in terms of adverse events averted over argatroban and bivalirudin (0.9994 vs 0.9965 and 0.9947).
Variable adverse events rates.
One-way sensitivity analysis showed that argatroban and bivalirudin would prevail over fondaparinux only in terms of adverse events averted if the baseline rates of HIT-related VTE and major bleeding decreased by ≥75% for argatroban (2.84% and 1.56%, respectively) and ≥80% for bivalirudin (0.91% and 1.52%, respectively). For this analysis, fondaparinux baseline rates of HIT-related VTE and major bleeding remain at 11.36% and 2.3%, respectively. The incremental cost-effectiveness ratio (ICER) for argatroban compared with fondaparinux would be $12 729 883, and the ICER for bivalirudin compared with fondaparinux would be $13 005 514. Even with substantial improvements in the efficacy profile of argatroban and bivalirudin, our study indicates that fondaparinux would prevail in terms of cost savings utilizing current local prices.
Discussion
We describe here in the first pharmacoeconomic comparison from the institutional perspective of argatroban, bivalirudin, and fondaparinux for the management of suspected HIT. We found that fondaparinux prevailed over argatroban and bivalirudin in all scenarios tested. Fondaparinux was cost-effective compared with both of the other treatment options, having a much lower cost when both institutional and AWP costs were used. Using institutional costs, argatroban outperformed bivalirudin in terms of cost savings and adverse events averted; however, argatroban was more expensive while maintaining a higher rate of adverse events averted using AWP. Further, the cost-effectiveness advantage of fondaparinux was confirmed in the additional analyses incorporating longer duration of nonheparin anticoagulants and lower incidence of HIT, suggesting the validity of our results across a range of model assumptions. In fact, 1-way sensitivity analysis on the efficacy side showed that the baseline rates of adverse events associated with argatroban and bivalirudin need to improve by at least 75% and 80%, respectively, for these agents to prevail over fondaparinux in terms of adverse events averted. In keeping with common pharmacoeconomic practice, the ICER was not calculated in the primary analysis, since fondaparinux was the dominant agent. Based on our results, fondaparinux provides an attractive clinical and economic alternative to argatroban and bivalirudin, affording large reductions in cost and the potential clinical benefit of reduced adverse effects. Our data suggest that greater consideration should be given to fondaparinux as a first-line agent for treatment of suspected HIT.
Our aim was to provide cost-effectiveness results using, in first instance, those determinants of costs that varied across clinical scenarios and treatment options. Where not constant for each of the 3 agents, estimates could be supported with sources from published literature and are influenced by local health care market and labor force dynamics. Technically, in doing so, we disfavored from the onset fondaparinux as the lowest cost agent over the 2 more expensive agents. This enabled us to derive highly conservative estimates, but with the caveat that our results may underestimate the cost-effectiveness of fondaparinux over argatroban and bivalirudin if other cost determinants were considered (an issue we address below). Additional strengths of our study include the use of recent cost data and incorporating standard clinical practices for HIT treatment and including the costs of dose titration and laboratory monitoring.
Both argatroban and bivalirudin have relatively short half-lives and are administered as continuous titratable infusions. The shorter half-lives offer the advantage of a more rapid elimination of anticoagulant effect in patients at risk of bleeding or requiring invasive procedures. Argatroban may also provide an advantage over bivalirudin or fondaparinux in the setting of dynamic renal function, since renal adjustment is not required for this hepatically cleared compound. Adjustment in the setting of hepatic dysfunction is recommended.39,48,49 Despite these advantages, argatroban and bivalirudin require IV access, increased nursing labor, regular blood draws for therapeutic monitoring, as well as pharmacy consultation for dosing and compounding. Calculated dose adjustments also increase the risk of dosing errors. In fact, frequent aPTT monitoring may result in incorrect dose adjustments and may possibly worsen outcomes, commonly known as “aPTT confounding.”50 Specifically, aPTT can be confounded by coagulopathy either due to comorbidity (eg, liver disease) or severe HIT itself. Potentially, this could lead to underdosing of nonheparin anticoagulant and may explain the failure of therapy to prevent thrombosis and amputation reported in pivotal trials. For argatroban, transition to warfarin is more challenging, since argatroban artificially increases the INR.14
Fondaparinux offers several clinical benefits over the other 2 agents studied. Its relatively simple administration reduces the indirect costs incurred in preparing, administering, and monitoring use. Fondaparinux allows easy conversion to warfarin and administration as outpatient treatment, which potentially reduces costs of inpatient treatment even further. This is a prominent advantage, especially for patients with persistent HIT, where platelets may take weeks to recover and fondaparinux is the only drug studied that provides the option of ongoing outpatient treatment of these patients. This advantage further underscores the cost difference between fondaparinux and the other 2 agents. We chose not to account for indirect medical costs, which are difficult to quantify accurately and sensitive to local health care market and labor force dynamics; for instance, nursing care related to ongoing IV infusion with dose titration and institutional differences in laboratory monitoring costs. Such indirect costs would not fundamentally alter, and only expand, the already established significant cost-effectiveness of fondaparinux over the other agents. Institutions considering adopting fondaparinux as a first-line agent for the treatment of suspected HIT may want to apply our estimates to their local costs.
Our results extend, from an economic point of view, the clinical findings from recent retrospective clinical studies17 and expert opinion3,18,-20 that fondaparinux may be a safe and effective option for the management of HIT. The largest trial to date comparing argatroban and fondaparinux in the management of HIT was performed by Kang et al as a retrospective study of nonheparin anticoagulant therapy in 133 fondaparinux-treated patients propensity-matched to 60 patients administered danaparoid or argatroban. They found no statistically significant differences in the rates of thrombosis and thrombosis-related mortality (respectively, 16.5% vs 21.4%; P = .424) and bleeding and bleeding-related mortality (resp., 21% vs 20%; P = .867) between the 2 groups. The authors concluded that fondaparinux is noninferior to argatroban and danaparoid for management of suspected HIT.17 In a case series of 16 patients, Warkentin et al offered encouraging findings when using fondaparinux in patients varying in renal function.21
Although these results suggest that fondaparinux may be used safely and effectively for suspected HIT, prospective head-to-head trials are needed to confidently evaluate the relative clinical benefits of fondaparinux vs FDA-approved therapies. The critical need for these trials is underscored by the large potential cost savings afforded with fondaparinux treatment. In an effort to error on the conservative side of savings, many of our assumptions favored argatroban and bivalirudin ab initio. We did not account for pharmacist time spent compounding both agents; clinical pharmacist consult time for dosing, IV compatibility, and dose titration; establishing IV access and calculating dose adjustments, IV bags, and tubing; whether new IV access was required specifically for infusion; the true cost of aPTT and INR monitoring throughout therapy; and the cost of drug waste, as these variables would have further disfavored both agents. We assumed an abbreviated course of therapy and a single aPTT based on an ideal scenario of readily reaching the therapeutic range target, a rapid recovery of platelets to >150 × 109/L, and quick transition to oral anticoagulant therapy. However, clinical practice often requires frequent aPTT monitoring while on argatroban or bivalirudin. A longer treatment duration of both drugs is likely to result in even greater total costs compared with fondaparinux. We did not assess patient satisfaction associated with any therapy because there are no validated preference data available for these anticoagulants. The use of general data on continuous IV therapy vs daily injection therapy would be speculative. We also did not assess the potential for earlier discharge while on fondaparinux, because this is likely to be influenced by local practice. These are clinically important factors; however, they would further disfavor argatroban and bivalirudin in terms of cost-effectiveness.
Our study has limitations. The assumed rates of complications associated with the use of anticoagulants were based on published literature. This literature consists of relatively small trials with a range of complications reported but constitutes the best available evidence for these complication rates.7,17,24,25,34,35 In clinical practice, comorbidities and other patient-specific risk factors may result in different risks of VTE or bleeding complications. In the absence of a definite cost of a major bleeding episode, we used the cost of managing GI bleeding as an estimated cost of major bleeding.
The relative lack of data evaluating amputation risk associated with fondaparinux in HIT patients precluded our ability to include this outcome. Furthermore, including amputation would disfavor argatroban significantly more; whereas, excluding amputation is in line with our aim to derive conservative estimates. Evaluating the all-cause amputation cases reported in the argatroban pivotal trials, Haas et al reported that 97.5% of amputation cases were diagnosed with ischemia/gangrene before argatroban was initiated.33 Also, about 46% of amputated patients were treated with warfarin prior to argatroban. Thus, the risk of amputation attributable to argatroban may have been overestimated in these trials. It should be noted that the results of this study do not necessarily rule out that argatroban could worsen a case of HIT especially if viewed in light of aPTT confounding.
Given the cost-effectiveness of fondaparinux, further evaluation of fondaparinux as first-line treatment of HIT over argatroban and bivalirudin is warranted. Our study underscores the need for head-to-head comparisons between fondaparinux, argatroban, and bivalirudin, which would provide valuable information to judge the place of fondaparinux in HIT treatment. Fondaparinux should also be considered in evaluations of new oral anticoagulants, which have an emerging role in the management of HIT. The benefits observed in our study are likely to persist despite patent expiration and cost reduction due to generic competition for argatroban, considering the substantial differences in current drug cost. On-demand rather than batched-HIT testing has been proposed as a strategy to reduce time to confirmation of the diagnosis of HIT. Since assumptions are that 2 days for return of HIT antibody testing, on-demand HIT testing may change the clinical picture of our assumptions; although, again, this is unlikely to change fondaparinux’s cost-effectiveness.51
In conclusion, to our knowledge, this is the first cost-effectiveness analysis comparing treatment-dose fondaparinux, argatroban, and bivalirudin for the management of suspected HIT. Considering the 3 agents’ efficacy and safety profiles, the cost savings and adverse events averted by a once-daily fondaparinux subcutaneous injection with limited laboratory monitoring are more advantageous than continuous titration of argatroban or bivalirudin infusions. Our data strongly suggest that, in the United States, fondaparinux is a cost-responsible approach to the management of suspected heparin-induced thrombocytopenia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank James Camamo for his efforts in calculating AWP. They also also thank anonymous reviewers for the suggestion to include additional analyses with different HIT incidence rates and durations of therapy, as well as sensitivity analyses on the efficacy side.
The authors thank the members of the postdoctoral fellowship programs in (1) Clinical Research in Human Therapeutics and (2) Health Outcomes, Effectiveness, and Economics Research at the University of Arizona for their comments on this study; these programs funded the work reported here. J.H.K. is supported by a Futures Grant from the American College of Clinical Pharmacy Research Institute (Lenexa, KS).
Authorship
Contribution: A.A., B.E., H.I.K., I.A., M.G., S.Y., and Y.H. designed the study; A.A., H.I.K., I.A., and J.H.K. were responsible for data abstraction; A.A., I.A., and M.G. were responsible for economic modeling; A.A., I.A., J.H.K., Y.H. wrote the first draft of the manuscript; and A.A., B.E., H.I.K., I.A., J.H.K., M.G., S.Y., Y.H., and Y.R. reviewed the manuscript for scientific content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ivo Abraham, Center for Health Outcomes and PharmacoEconomic Research, University of Arizona, Drachman Hall B-306, 1295 N Martin, Tucson, AZ 85721; e-mail: abraham@pharmacy.arizona.edu.