To the editor:
Hexokinase (HK) deficiency is a very rare inherited disorder of the Embden-Meyerhof glycolytic pathway of erythrocytes where the predominant clinical presentation is congenital nonspherocytic hemolytic anemia. HK catalyzes the initial phosphorylation of glucose to glucose 6 phosphate and is considered 1 of the rate-limiting enzymes of red cell anaerobic glycolysis.1 Of all of the glycolytic enzymes, HK is the most influenced by erythrocyte age. Mature erythrocytes may have no more than 2% to 3% of their HK activity originally present in the reticulocytes.2,3 There are 4 isoenzymes of HK (HK I-IV) of which HK-I is the predominant isoenzyme in human tissues. Erythrocytes contain a specific subtype of HK (HK-R) that is encoded by the HK-I gene (HK1) localized on chromosome 10q22.4 Lymphocytes, platelets, and granulocytes depend more or less heavily on energy supplied by glycolysis; therefore, HK plays an important role in the energy metabolism of these cells too.5 The disease severity is variable, ranging from mild (where the hemolysis is fully compensated) to severe disease (presenting with hydrops fetalis, neonatal hyperbilirubinemia, and severe anemia) that requires chronic blood transfusions.6
We treated a 4-year-old Hispanic boy who was born at 32 weeks of gestation with hydrops fetalis, severe anemia (hemoglobin, 4.5 g/dL), and indirect hyperbilirubinemia. His family history was remarkable for 2 siblings who had died at a few hours of life secondary to severe anemia and hydrops. He required exchange transfusion (total serum bilirubin, 7.5 mg/dL in the first few hours of life). Prior to newborn discharge, he required packed red blood cell (PRBC) transfusion and continued to be PRBC transfusion dependent as an outpatient (on average every 4 weeks with goal hemoglobin level, >8 g/dL).
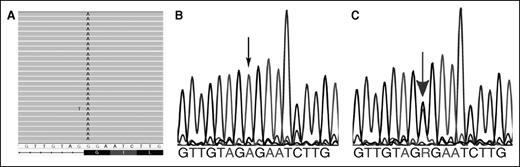
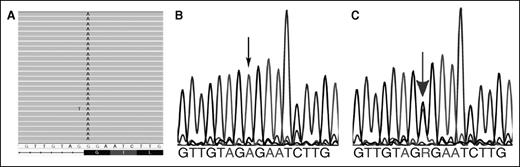
Evaluation of his transfusion-dependent anemia by whole-exome sequencing7 revealed a novel mutation with homozygous nucleotide substitution in the first nucleotide of exon 13 of the HK 1 gene (NM_000188:c.G1840A:p.G614R; chr10:7116079, hg19) leading to both Gly614Arg, a missense mutation predicted to be severely damaging, and disruption of normal splicing at the 3′ acceptor splice site, ag:G to ag:A (Figure 1).
Mutation identification. (A) Exome sequencing. Graphical representation of sequence tags from exome sequencing (HX proband, exon 13). All 26 full-length reads have an A instead of a G, leading to a missense mutation, Gly to Arg at amino acid 614, which also disrupts the normal splicing at the 3′ acceptor splice site of the exon, ag:G to ag:A. (B) Sanger sequencing confirmation of HK1 gene mutations (proband). The corresponding partial exon 13 sequence from the proband reveals only homozygous “A” at the beginning of the exon, denoted by the small black arrow. (C) Sanger sequencing confirmation of HK1 gene mutations (parents). The corresponding partial exon 13 sequence from the mother reveals both “A” and “G” alleles, denoted by the large gray arrow, indicating the presence of wild-type and mutant alleles. Similar findings were observed in the father (not shown).
Mutation identification. (A) Exome sequencing. Graphical representation of sequence tags from exome sequencing (HX proband, exon 13). All 26 full-length reads have an A instead of a G, leading to a missense mutation, Gly to Arg at amino acid 614, which also disrupts the normal splicing at the 3′ acceptor splice site of the exon, ag:G to ag:A. (B) Sanger sequencing confirmation of HK1 gene mutations (proband). The corresponding partial exon 13 sequence from the proband reveals only homozygous “A” at the beginning of the exon, denoted by the small black arrow. (C) Sanger sequencing confirmation of HK1 gene mutations (parents). The corresponding partial exon 13 sequence from the mother reveals both “A” and “G” alleles, denoted by the large gray arrow, indicating the presence of wild-type and mutant alleles. Similar findings were observed in the father (not shown).
He was diagnosed with congenital nonspherocytic hemolytic anemia secondary to HK deficiency and underwent a laparoscopic splenectomy in the second year of life in an attempt to reduce transfusion requirements and iron overload. Following splenectomy, his transfusion frequency decreased to every 2 to 3 months, permitting confirmation of decreased HK activity (0.7 U/g hemoglobin [normal range, 0.8-1.9]). He continued to require PRBC transfusions and was started on chelation therapy with deferoxamine at the age of 2 years for significant iron overload (11.6 mg iron per gram liver dry weight). He responded well to the decreased frequency of transfusions and ongoing chelation, with a liver iron concentration of 4.6 mg iron per gram liver dry weight by the age of 3 years.
The patient had an unaffected histocompatible sibling donor matched at HLA A, B, C, DRB1, and DQ. He was referred to the Blood and Marrow Transplantation (BMT) Program at Children’s Hospital at Montefiore for evaluation. Pre-BMT assessment was significant for Epstein-Barr viremia which took 4 weeks to clear.
A family conference was held to discuss the risks and benefits of BMT. A reduced-intensity strategy vs a more intensive myeloablative approach was discussed. The family consented to BMT using a reduced-intensity approach. He received a conditioning regimen consisting of proximal (day −14 to day −10) IV alemtuzumab (1 mg/kg), fludarabine (150 mg/m2 IV), and melphalan IV (140 mg/m2)7 followed by a 10 of 10 matched related, ABO-compatible, sex-matched BMT (bone marrow; total nucleated cells, 6 × 108/kg; CD34 cells, 6.7 × 106/kg; and CD3 cells, 5.7 × 107/kg). Graft-versus-host disease prophylaxis consisted of tacrolimus (started day −2) and methylprednisolone (started on day −14).
He achieved neutrophil and platelet recovery (20 000/mm3) on day +17 and day +19, respectively. Initial sorted short tandem repeats nucleated cell chimerism analysis was 98% donor in whole blood and the CD33 fraction; other fractions were not reported due to insufficient sample. Repeat sorted nucleated cell chimerism (day +32) was of donor origin in 15% of the CD3 fraction and 50% in the CD33 fraction. Mature erythrocyte chimerism was not assessed. Immune suppression was discontinued on day +40. His posttransplant course was also complicated by asymptomatic cytomegalovirus reactivation on day +1 and he was managed with IV foscarnet for 6 weeks. He also developed asymptomatic Epstein-Barr viremia, which persisted for 6 months post-BMT. He was treated with oral valganciclovir for 6 weeks and then maintained on acyclovir prophylaxis dosing. No sinusoidal obstruction syndrome or graft-versus-host disease was observed. T-lymphocyte (CD3+) and NK-cell (CD3−, CD16+, CD56+) recovery >50/mm3 was achieved by day +35; B-lymphocyte (CD19+) recovery >50/mm3 by day +140. Eight months after transplant, red cell HK activity was normal (1.7 U/g hemoglobin). The patient is >1 year posttransplant and he remains transfusion independent (last PRBC transfusion was on 1 occasion post-BMT on day +12), with persistent mixed donor chimerism and hemoglobin at 9 g/dL. His current Lansky performance score is 100. His only medication is a multivitamin and folic acid and he has not developed viral reactivation. His iron overload status has improved significantly without chelation (1.7 mg iron per gram liver dry weight).
To our knowledge, this is the first reported case of allogeneic BMT for a patient with hemolytic anemia secondary to HK deficiency that resulted from a novel genetic mutation. Rhodes at al reported a case of successful BMT in a patient with phosphoglycerate kinase deficiency using myeloablative conditioning with busulfan, cyclophosphamide, and thymoglobulin.8 Tanphaichitr et al reported a case of successful BMT using myeloablative conditioning (busulfan/cyclophosphamide) that resulted in full donor engraftment and cure of a 4-year-old child with pyruvate kinase (PK) deficiency.9 Animal studies have shown that establishment of mixed donor chimerism in PK-deficient dogs using a nonmyeloablative conditioning regimen (200 cGy total body irradiation) and BMT can be accomplished, but mixed chimerism was not sufficient to completely eliminate hemolysis. Therefore, conversion to all donor chimerism appeared to be necessary for a long-term cure for hemolytic anemia due to PK deficiency in dogs.10 However, Andreani et al have shown that among transplanted patients with β-thalassemia major and sickle cell disease with persistent mixed chimerism, despite the presence of a few donor nucleated cells in the peripheral blood and bone marrow, the erythrocytes were almost completely of donor origin.11 Why, among some patients, mixed chimerism represents a transient condition, whereas among others the coexistence of donor and recipient cells persist through the entire posttransplant follow-up, is still unknown.12
The risks and benefits of a myeloablative vs reduced-intensity preparative regimen were considered. Based on successful outcomes for other nonmalignant diseases using a reduced-intensity approach with potentially less long-term toxicity, the family opted for this approach. Although the patient remains transfusion-independent >1 year post-BMT, his dropping donor chimerism remains concerning for graft rejection. Future studies are needed to elucidate optimal conditioning strategies for this disease as well as the utility of mature erythrocyte donor chimerism testing. However, we establish that allogeneic BMT is a potentially curative strategy for this disease.
Authorship
Contribution: S.K. completed the data collection and wrote the manuscript; V.P. assisted in writing the manuscript; D.M. diagnosed the patient and assisted in editing the manuscript; P.G.G. performed the genetic testing and edited the manuscript; S.P. assisted in data collection; and K.M.M. developed the treatment regimen, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sajad Khazal, Pediatric Marrow and Blood Cell Transplantation Program, Children’s Hospital at Montefiore, Albert Einstein College of Medicine, 3415 Bainbridge Ave, Bronx, NY 10467; e-mail: skhazal@montefiore.org.