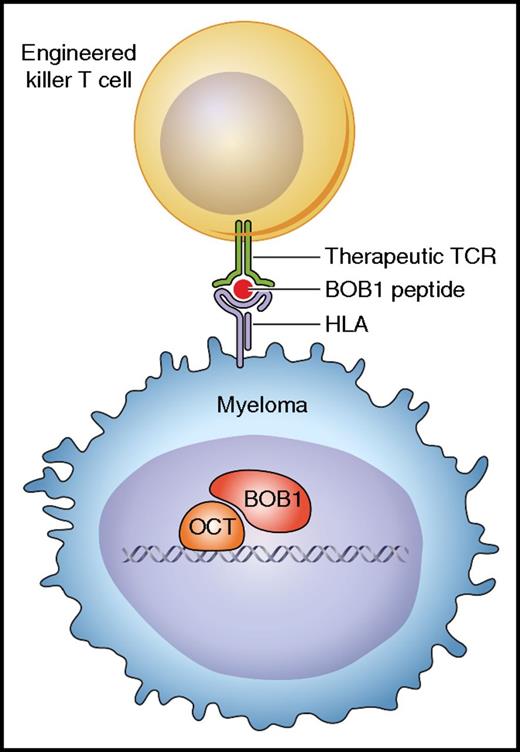
In this issue of Blood, Jahn and colleagues have demonstrated that T-cell receptor (TCR) gene transfer can produce cytotoxic T cells that are capable of eliminating myeloma cells in vivo.1 The therapeutic TCR can equip human T cells with specificity for octamer binding protein-1 (BOB1), a transcription coactivator that is expressed in normal B cells, multiple myeloma, and B-lineage malignancies, thus providing novel options for immunotherapy of B-cell tumors that do not express CD19 or other surface antigens suitable for targeting with chimeric antigen receptor (CAR)–engineered T cells (see figure).
Cytotoxic T cells engineered with the BOB1-specific TCR can recognize and kill myeloma cells expressing the BOB1 transcriptional coactivator intracellularly. The TCR is specific for BOB1 peptides presented by HLA class I molecules. Professional illustration by Patrick Lane, ScEYEnce Studios.
Cytotoxic T cells engineered with the BOB1-specific TCR can recognize and kill myeloma cells expressing the BOB1 transcriptional coactivator intracellularly. The TCR is specific for BOB1 peptides presented by HLA class I molecules. Professional illustration by Patrick Lane, ScEYEnce Studios.
The clinical success of immunotherapy with gene-modified T cells targeting the CD19 molecule has caused excitement among patients, clinicians, and scientists, and has resulted in substantial investment from industry over the past years.2 CD19 is a cell surface molecule that is expressed in a large proportion of normal and malignant B cells and is efficiently recognized by CARs. A major advantage of CAR-engineered T cells is the direct recognition of the CD19 molecule, which enables treatment of patients independent of their HLA genotype. A limitation of this form of immunotherapy is that a number of B-cell malignancies, such as multiple myeloma, do not express the CD19 target antigen. Jahn and colleagues have therefore explored whether TCR gene therapy can provide a novel treatment option for all B-cell tumors. Unlike CARs, which require cell surface expression of their target antigen, the HLA-restricted mechanism of antigen recognition enables TCRs to target any intracellular protein. Thus, TCR gene therapy provides access to a large number of intracellular targets that are not accessible for therapy with the CAR technology.
In an elegant piece of research, Jahn et al first screened the expression profiles of normal and malignant B cells to identify POU2AF1 as a gene with a B-lineage–specific expression profile. The POU2AF1 gene encodes BOB1 (also known as OCA-B or OBF), which acts in conjunction with the OCT1 and OCT2 transcription factors to activate gene expression.3 In addition, BOB1 binding to the tyrosine kinase SYK in the cytosol of B cells has been implicated in intracellular B-cell receptor signaling.4 Next, Jahn et al tested whether BOB1-derived peptides are naturally produced and presented to T cells by screening peptide libraries that were eluted from HLA class I molecules of B lymphoblastoid cell lines. They identified four BOB1 peptides, one naturally presented by HLA-A*0201 and three by HLA-B*07.02. All four peptides were used to generate HLA/peptide tetramer reagents to stain T cells from healthy donors and purify BOB1-specific T cells by single-cell sorting. The use of donors who were negative for HLA-A*0201 and HLA-B*07.02 provided a strategy to circumvent T-cell tolerance to BOB1 peptides presented by these HLA alleles.5 One HLA-B*07.02–restricted T-cell clone displayed a high level of BOB1 specificity, killing primary tumor cells of patients with chronic lymphocytic leukemia (CLL), acute lymphoblastic leukemia (ALL), mantle cell lymphoma, and multiple myeloma. This clone was used to isolate the genes encoding TCR α and β chains in order to generate retroviral vectors for TCR gene transfer to equip peripheral-blood T cells with BOB1 specificity. As expected, the TCR gene–engineered T cells displayed the same BOB1 specificity as the original T-cell clone, and experiments in a xenogenic mouse model demonstrated that engineered T cells were able to control the growth of multiple myeloma cells in vivo.
The gene expression analyses of primary tumor cells suggest that BOB1 is present in ALL, CLL, and multiple myeloma but absent in myeloid malignancies such as acute myeloid leukemia and chronic myeloid leukemia. This indicates that TCR targeting of BOB1 provides a therapeutic option for treatment of B-lineage malignancies. The gene expression profiles of normal tissues indicate that BOB1 is absent in nonhematopoietic cells, and within the hematopoietic lineage its expression is restricted to the normal B-cell compartment. The side-effect profile of BOB1-specific TCR gene therapy is therefore expected to result in the depletion of the normal B-cell compartment, similar to what has been observed in patients treated with CAR-engineered T cells targeting CD19. The density of the BOB1 and CD19 target antigens in normal and malignant B cells is expected to be vastly different. Whereas BOB1 peptides presented by HLA class I molecules may reach a density of 10 to 100 epitopes per cell, the CD19 expression is in the range of 10 000 to 100 000 per cell. It is possible that the high antigen density results in exaggerated T-cell activation, which may contribute to cytokine storm and the associated toxicity that is observed in patients after treatment with CD19-CAR–engineered T cells. It will be interesting to explore whether the lower antigen density of BOB1 combined with physiologic stimulation via the TCR is associated with less aggressive T-cell activation that is sufficient to achieve tumor protection while reducing the risk of cytokine storm.
In summary, BOB1 has been validated as an attractive target for TCR gene therapy of multiple myeloma and other B-cell malignancies. The preclinical data provide a solid basis to test this TCR in clinical trials to determine its therapeutic efficacy and the side-effect profile.
Conflict-of-interest disclosure: H.J.S. is advisor and shareholder of Cell Medica and receives research funding from this biotech company.