To the editor:
Mutations in the transcription factor Growth Factor Independent1B (GFI1B) are causal to an autosomal dominant inherited bleeding disorder characterized by macrothrombocytopenia and reduced platelet α-granule numbers (platelet-type bleeding disorder-17, Online Mendelian Inheritance in Man [OMIM] #187900).1-3 The reduction in α-granules causes a gray appearance of platelets on May-Grünwald-Giemsa (MGG)-stained blood films. In addition to these abnormalities, we reported expression of the hematopoietic stem and progenitor marker CD34 on platelets (and megakaryocytes [MKs]) from affected family members (GFI1B p.Q287*).1 This finding was recently confirmed in a family with a different GFI1B truncating mutation (p.G272fs).3 Based on these results, we routinely examine platelet CD34 expression in the diagnostic work-up of unexplained inherited bleeding disorders. We observed platelet CD34 expression in a family with an autosomal dominant inherited bleeding disorder with thrombocytopenia, and 2% to 14% gray platelets in MGG-stained blood films (Figure 1A-B,D [left]; see supplemental Figure 1A, available on the Blood Web site; and RUNX1 p.Q154fs cases I:1 and II:1 to II:3 in Table 1).4 No abnormalities were detected in the coding region or exon boundaries of GFI1B using Sanger sequencing. Using whole exome sequencing and next-generation sequencing of a myeloid gene panel (Ion AmpliSeq AML panel; Life Technologies), we detected and confirmed a heterozygous mutation causing a frameshift in the transcription factor RUNX1 (p.Q154fs; Figure 1C). This mutation is situated in the DNA binding Runt domain and was detected in all tested thrombocytopenic family members. Similar RUNX1 mutations have been described in the autosomal dominant inherited bleeding disorder FPDMM (familial platelet disorder with associated myeloid malignancy, OMIM #601399). Although myeloid malignancies have not been documented in this family, the mother of case I:1 had a history of thrombocytopenia and developed non-Hodgkin’s lymphoma at 50 years of age. Platelet CD34 expression has not been reported before for RUNX1-mutated cases. To study whether other bleeding disorder-associated RUNX1 mutations cause platelet CD34 expression, we studied platelets from 2 thrombocytopenic siblings from a family with a heterozygous tandem duplication of RUNX1 exons 2-6 that we reported earlier5 (RUNX1 TD exons 2-6 cases II:3 and II:6 in Table 1). Platelets from these individuals were also CD34 positive (Figure 1D, left), indicating that platelet CD34 expression occurs in both GFI1B- and RUNX1-related bleeding disorders. The specificity of these findings was illustrated by the absence of platelet CD34 expression in 11 other cases of inherited thrombocytopenia (n = 5; supplemental Table 1) or immune thrombocytopenia purpura (n = 6; 3 acute and 3 chronic cases, data not shown).
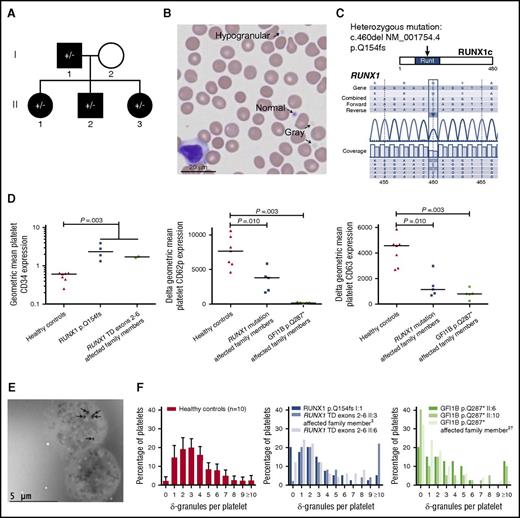
CD34 expression and granule contents of RUNX1- and GFI1B-mutated platelets. (A) Pedigree of the family harboring a heterozygous RUNX1 p.Q154fs mutation (indicated by ±). Thrombocytopenia is indicated with black shapes. Also the deceased mother of case I:1 had a history of thrombocytopenia (data not shown), suggesting autosomal dominant inheritance of the disease. Four family members were screened for the mutation (all ±). □: male, ○: female. Pedigrees from the RUNX1 tandem duplication exons 2-6 and GFI1B p.Q287* families were published before.1,5 (B) MGG-stained blood film of proband I:1 (RUNX1 p.Q154fs) showing normal, hypogranular, and agranular (gray) platelets (arrows). Original magnification ×40, VisionTek Sakura, VisionTek Live 2.6. (C) Sequencing of RUNX1 revealed a heterozygous mutation (c.460del NM_001754.4, p.Q154fs) in all tested affected family members. Results from the proband I:1 are shown. (D) Geometric MFI of CD34 expression on CD41-positive platelets measured by flow cytometry showing that thrombocytopenic individuals from RUNX1-mutated families (p.Q154fs cases I:1, II:1-II:3, and tandem duplication exons 2-6 cases II:3 and II:6) have higher platelet CD34 expression than healthy controls. The line indicates the median (left). Geometric MFI of CD62p on CD42a-positive (middle) and CD63 on CD42b-positive (right) platelets (thrombin-stimulated minus unstimulated). On average, individuals from RUNX1-mutated families (p.Q154fs cases I:1, II:2, II:3, and tandem duplication exons 2-6 cases II:3 and II:6) and the GFI1B p.Q287*-mutated family (cases II:6, II:10, and the last case from Table 1, plus cases II:3 and II:8 from Monteferrario et al1 ) had decreased CD62p and CD63 expression compared with healthy controls. (E) Whole mount EM on platelets from proband I:1. All δ-granules are indicated with arrows. The lower of the two platelets is devoid of δ-granules. Original magnification ×1200, Jeol JEM 1400, Gatan digital micrograph software. (F) Quantification of δ-granules using whole mount EM. The number of δ-granules was determined for 40 or 50 platelets (counters were blinded to diagnosis). As reference, the left panel shows the percentage of platelets harboring 0 to 9 or ≥10 δ-granules for healthy controls (n = 10, each bar with whisker shows mean + 99.55% CI). The middle and right panels show that 2 of 3 thrombocytopenic individuals from RUNX1-mutated families and 3 of 3 from GFI1B p.Q287*-mutated families had an increased percentage of platelets harboring no δ-granules (affected: 12% to 40%; healthy controls: 0% to 4.4% with a 99.55% CI). The median number of δ-granules per platelet was significantly decreased for GFI1B p.Q287* cases compared with controls (P = .013). Because results from the 3 individuals from RUNX1-mutated families were not consistent, the median number of δ-granules per platelet was not significantly decreased for these cases compared with controls (P = .08). One of 3 and 2 of 3 individuals from RUNX1- and GFI1B-mutated families, respectively, had increased percentages of platelets containing ≥10 δ-granules (affected: 10% to 22%; healthy controls: 0% to 5.2% with a 99.55% CI). ‡These individuals were thrombocytopenic, but not tested for the indicated mutation. †This case is a son of case II:10 and nephew of II:6, but was not included in the previous publication.1 All P values were obtained using the 2-sided Mann-Whitney U test. CI, confidence interval; MFI, mean fluorescence intensity; TD, tandem duplication.
CD34 expression and granule contents of RUNX1- and GFI1B-mutated platelets. (A) Pedigree of the family harboring a heterozygous RUNX1 p.Q154fs mutation (indicated by ±). Thrombocytopenia is indicated with black shapes. Also the deceased mother of case I:1 had a history of thrombocytopenia (data not shown), suggesting autosomal dominant inheritance of the disease. Four family members were screened for the mutation (all ±). □: male, ○: female. Pedigrees from the RUNX1 tandem duplication exons 2-6 and GFI1B p.Q287* families were published before.1,5 (B) MGG-stained blood film of proband I:1 (RUNX1 p.Q154fs) showing normal, hypogranular, and agranular (gray) platelets (arrows). Original magnification ×40, VisionTek Sakura, VisionTek Live 2.6. (C) Sequencing of RUNX1 revealed a heterozygous mutation (c.460del NM_001754.4, p.Q154fs) in all tested affected family members. Results from the proband I:1 are shown. (D) Geometric MFI of CD34 expression on CD41-positive platelets measured by flow cytometry showing that thrombocytopenic individuals from RUNX1-mutated families (p.Q154fs cases I:1, II:1-II:3, and tandem duplication exons 2-6 cases II:3 and II:6) have higher platelet CD34 expression than healthy controls. The line indicates the median (left). Geometric MFI of CD62p on CD42a-positive (middle) and CD63 on CD42b-positive (right) platelets (thrombin-stimulated minus unstimulated). On average, individuals from RUNX1-mutated families (p.Q154fs cases I:1, II:2, II:3, and tandem duplication exons 2-6 cases II:3 and II:6) and the GFI1B p.Q287*-mutated family (cases II:6, II:10, and the last case from Table 1, plus cases II:3 and II:8 from Monteferrario et al1 ) had decreased CD62p and CD63 expression compared with healthy controls. (E) Whole mount EM on platelets from proband I:1. All δ-granules are indicated with arrows. The lower of the two platelets is devoid of δ-granules. Original magnification ×1200, Jeol JEM 1400, Gatan digital micrograph software. (F) Quantification of δ-granules using whole mount EM. The number of δ-granules was determined for 40 or 50 platelets (counters were blinded to diagnosis). As reference, the left panel shows the percentage of platelets harboring 0 to 9 or ≥10 δ-granules for healthy controls (n = 10, each bar with whisker shows mean + 99.55% CI). The middle and right panels show that 2 of 3 thrombocytopenic individuals from RUNX1-mutated families and 3 of 3 from GFI1B p.Q287*-mutated families had an increased percentage of platelets harboring no δ-granules (affected: 12% to 40%; healthy controls: 0% to 4.4% with a 99.55% CI). The median number of δ-granules per platelet was significantly decreased for GFI1B p.Q287* cases compared with controls (P = .013). Because results from the 3 individuals from RUNX1-mutated families were not consistent, the median number of δ-granules per platelet was not significantly decreased for these cases compared with controls (P = .08). One of 3 and 2 of 3 individuals from RUNX1- and GFI1B-mutated families, respectively, had increased percentages of platelets containing ≥10 δ-granules (affected: 10% to 22%; healthy controls: 0% to 5.2% with a 99.55% CI). ‡These individuals were thrombocytopenic, but not tested for the indicated mutation. †This case is a son of case II:10 and nephew of II:6, but was not included in the previous publication.1 All P values were obtained using the 2-sided Mann-Whitney U test. CI, confidence interval; MFI, mean fluorescence intensity; TD, tandem duplication.
To determine whether RUNX1- and GFI1B-related inherited platelet disorders have more abnormalities in common, we measured platelet granule content. GFI1B-mutated individuals have decreased platelet α-granules.1-3 To study α-granule content in RUNX1- and GFI1B-mutated platelets, we performed CD62p (P-selectin) flow cytometry following thrombin activation, platelet factor 4 (PF4) and β-thromboglobulin (β-TG) enzyme-linked immunosorbent assays, and electron microscopy (EM). The geometric mean fluorescent intensity (MFI) of platelet CD62p and the percentage of CD62p-positive platelets were significantly lower in individuals from RUNX1- and GFI1B-mutated families compared with controls (Figure 1D, middle and supplemental Figure 1B). Although the CD62p expression was clearly lower in all cases with the GFI1B p.Q287* mutation, some RUNX1 affected cases exhibited values similar to that observed in healthy controls. PF4 and β-TG are low in GFI1B-mutated platelets (GFI1B p.Q287* cases II:6, II:10 and the last case from Table 1).1 In affected members of RUNX1-mutated families, the expression of PF4 and β-TG varied in time, being diminished in 3 of 6 (PF4) and 5 of 6 (β-TG) thrombocytopenic individuals at least at one point in time (Table 1). EM analysis showed decreased α-granules in ∼65% of RUNX1 p.Q154fs-mutated platelets from the proband I:1 (supplemental Figure 1C), compared with ∼75% to 90% of GFI1B p.Q287*-mutated platelets. Thus, the expression of the α-granule membrane marker CD62p, PF4, β-TG, and EM analyses indicate a reduction in α-granules and/or its contents in RUNX1- and GFI1B-affected platelets. Reduced numbers of platelet α-granules analyzed by EM have sporadically been reported for FPDMM cases with mutations affecting different RUNX1 regions (p.T219fs6 and p.Y260*7 ). In conclusion, mutations in different domains of RUNX1 may cause a decrease in platelet α-granules.
Reductions in platelet δ-granule content have also been reported in RUNX1-mutated platelets.6,8,9 Platelet δ-granule numbers appeared normal by EM in the family with the GFI1B p.Q287* mutation that we reported earlier.1 Because platelet sections were analyzed with this approach, it was not suitable for very accurate quantification of δ-granule numbers, as the total number per platelet is low. To quantify δ-granules more reliably, we analyzed entire platelets by whole mount EM, in which δ-granules appear as black spots due to their high calcium content (Figure 1E and supplemental Figure 1E). This showed reduced δ-granule numbers in platelets from 2 of 3 RUNX1 and 3 of 3 GFI1B affected family members compared with healthy individuals; in healthy controls, we observed that 0% to 4.4% of platelets completely lacked δ-granules, whereas this was 12% to 40% for 2 of 3 RUNX1 and 3 of 3 GFI1B affected family members (Figure 1F). In line with these observations, the expression of the δ-granule and lysosomal membrane marker CD63 and platelet content of the δ-granule component ADP were low and ATP/ADP ratios were high in RUNX1-mutated and GFI1B p.Q287* platelets compared with normal platelets (Figure 1D, right; supplemental Figure 1D; Table 1). Remarkably, 1 of 3 and 2 of 3 individuals from RUNX1- and GFI1B-mutated families, respectively, had increased percentages of platelets containing ≥10 δ-granules (10% to 22% in affected vs 0% to 5.2% in healthy controls; Figure 1F; supplemental Figure 1E). For GFI1B-mutated cases, this may be due to the presence of macroplatelets (Table 1), but abnormal granule routing could potentially also contribute to this phenomenon.
We conclude that inherited GFI1B and RUNX1 mutations are associated with platelet CD34 expression and that platelet α- and δ-granule numbers are often lower compared with those observed in normal platelets (hence explaining the gray appearance of platelets). Platelet CD34 expression in RUNX1-mutated families was somewhat unanticipated because RUNX1 stimulates CD34 expression in hematopoietic stem cells.10 The platelet CD34 expression observed here may be the result of defective MK maturation or RUNX1 itself may be involved in silencing CD34 expression during normal megakaryopoiesis. To gain more insight into the modulation of CD34 expression and to determine whether platelet CD34 expression is specific for RUNX1- and GFI1B-related bleeding and platelet disorders, platelet CD34 expression should be measured in platelet disorders caused by other (transcription factor) mutations. It would be interesting to test whether platelet CD34 expression can be used as a marker for genetic variants that are pathogenic (eg, for novel variants in GFI1B11 ). We recommend screening for RUNX1 and GFI1B mutations if there are indications for platelet α/δ-granule paucity and CD34 expression. When the genetic cause of a patient’s inherited thrombocytopenia is known, one may monitor more closely for specific disease characteristics, such as the development of myeloid malignancies in case of certain RUNX1 mutations.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors acknowledge the Landsteiner Foundation for Blood Transfusion Research for financial support to study GFI1B-related inherited thrombocytopenia (project 1531); B.A.P.L.-v.G. acknowledges Baxter and CSL Behring for unrestricted educational grants; and the authors thank Jeroen van der Laak and Ton de Haan for help with statistical analyses.
Contribution: A.E.M., W.L.v.H., B.A.P.L.-v.G., J.H.J., M.C.J., and B.A.v.d.R. designed and coordinated research; A.E.M., W.L.v.H., K.M.H., W.B., B.W., A.O.d.G., A.S., and F.P. collected and analyzed data; B.A.P.L.-v.G. and M.C.J. arranged for obtaining patient informed consent; and A.E.M. and B.A.v.d.R. wrote the manuscript that was critically revised by all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bert A. van der Reijden, Radboud Institute for Molecular Life Sciences, Radboud University Medical Center, Geert Grooteplein zuid 8, 6525 GA Nijmegen, The Netherlands; e-mail: bert.vanderreijden@radboudumc.nl.