Key Points
tPA activates the contact system, and PKal blockade enhances tPA-mediated thrombolysis.
PKal contributes to hemorrhagic transformation and cerebral edema in mice with acute stroke receiving tPA.
Abstract
Thrombolytic therapy using tissue plasminogen activator (tPA) in acute stroke is associated with increased risks of cerebral hemorrhagic transformation and angioedema. Although plasma kallikrein (PKal) has been implicated in contributing to both hematoma expansion and thrombosis in stroke, its role in the complications associated with the therapeutic use of tPA in stroke is not yet available. We investigated the effects of tPA on plasma prekallikrein (PPK) activation and the role of PKal on cerebral outcomes in a murine thrombotic stroke model treated with tPA. We show that tPA increases PKal activity in vitro in both murine and human plasma, via a factor XII (FXII)–dependent mechanism. Intravenous administration of tPA increased circulating PKal activity in mice. In mice with thrombotic occlusion of the middle cerebral artery, tPA administration increased brain hemorrhage transformation, infarct volume, and edema. These adverse effects of tPA were ameliorated in PPK (Klkb1)–deficient and FXII-deficient mice and in wild-type (WT) mice pretreated with a PKal inhibitor prior to tPA. tPA-induced brain hemisphere reperfusion after photothrombolic middle cerebral artery occlusion was increased in Klkb1−/− mice compared with WT mice. In addition, PKal inhibition reduced matrix metalloproteinase-9 activity in brain following stroke and tPA therapy. These data demonstrate that tPA activates PPK in plasma and PKal inhibition reduces cerebral complications associated with tPA-mediated thrombolysis in stroke.
Introduction
Recombinant tissue-type plasminogen activator (tPA) is the only approved pharmacological therapy for thrombotic stroke in the acute phase.1 Although tPA-mediated thrombolysis is the standard of care for ischemic stroke, the efficacy of this therapeutic approach remains a challenge and its use is limited by concerns related to increased risk of hemorrhagic transformation and angioedema, which can worsen clinical outcomes.2-4 The development of more effective and safer tPA-mediated thrombolytic therapies for stroke depends on understanding the effects of tPA beyond its well-established role in fibrinolysis.5-8
Plasmin has been implicated as an activator of the kallikrein-kinin system (KKS),9 which has multiple roles in vascular functions, hemostasis, and thrombosis. The actions of the KKS are mediated by plasma kallikrein (PKal), a serine protease derived from the abundant circulating zymogen plasma prekallikrein (PPK). Zymogen activation by the serine protease factor XIIa (FXIIa) cleaves PPK into disulfide-linked heavy and light chains of catalytically active PKal, which in turn cleaves FXII to FXIIa to provide positive feedback amplification of the KKS. PKal also cleaves high-molecular-weight kininogen (HK) to generate bradykinin, which can cause vascular hyperpermeability and edema. The KKS can also exert prothrombotic effects mediated by the generation of FXIIa, which activates FXI and the intrinsic coagulation cascade.10 In addition, we have previously shown that PKal impairs hemostasis by interfering with collagen-mediated platelet activation, which contributes to hematoma expansion in an experimental model of hemorrhagic stroke.11 The role of the KKS on vascular leakage, inflammation, and thrombosis has been demonstrated in animal models of acute stroke.12-15 Although the clinical significance of PKal and bradykinin in stroke and its cerebral complications is not yet available, these factors have been identified as clinically significant mediators of vasogenic edema associated with hereditary angioedema.16,17 In addition, the KKS has been implicated in orolingual angioedema that is associated with tPA-mediated thrombolysis.18-20
The effects of negatively charged surfaces and macromolecules on KKS activation are well established.10,21,22 Although, PKal has been implicated as a plasminogen activator23 and protease-mediated activation of FXII by plasmin has been reported,9,24 the potential physiological significance of the KKS to outcomes associated with tPA therapy is not yet available. We hypothesized that tPA may activate KKS, which could contribute to its adverse effects on intracerebral hemorrhage and also counteract tPA’s thrombolytic efficacy. This report investigates the role of PKal in the complications and efficacy of tPA therapy using a murine thrombotic stroke model.
Methods
A detailed description of the methods can be found in the supplemental Methods, available on the Blood Web site.
Middle cerebral artery occlusion (MCAO) with photothrombotic laser model
Adult male wild-type (WT) C57BL/6J (Jackson Laboratory, Bar Harbor, ME), PPK gene-deficient mice (Klkb1−/−)11 and FXII gene-deficient mice (FXII−/−) (kindly provided by Francis J. Castellino, University of Notre Dame) weighing 24 to 28 g were used in this study. Photothrombotic laser model was produced as described elsewhere.8 We performed all experiments in accordance with the guidelines of National Institutes of Health, Guide for the Care and Use of Laboratory Animals, and with approval from the Animal Care and Use Committee of Joslin Diabetes Center.
tPA administration
Two hours after MCAO, thrombolysis was initiated by jugular vein administration of tPA (10 mg/kg, Cathflo Activase; Genentech, South San Francisco, CA) in saline, with 10% being given as a bolus and the remaining 90% infused at 3 μL/min over 30 minutes. A selective PKal inhibitor BPCCB (1-benzyl-1H-pyrazole-4-carboxylic acid 4-carbamimidoyl-benzylamide)25,26 in 10% PEG400 in saline at doses of 1, 3, and 10 mg/kg was administered in a total volume of 100 μL, as a 50 μL bolus and 50 μL infused at 2 μL/min over 15 minutes before tPA infusion. Recombinant tPA (10 mg/kg) was administered at a dose typically used in rodents,8,27-29 which is higher than the clinical dose of 0.9 mg/kg in humans. The dose 10 mg/kg has been shown to be more effective than 0.9 mg/kg in mediating thrombolysis in mice.30
Kallikrein-like activity
Citrated plasma was incubated with purified human tPA (0 to 360 nM) for 10 minutes. Kallikrein-like activity was determined by measurement of the changes in fluorescence at 410 nm because of hydrolysis of 100 μmol/L d-Pro-Phe-Arg-7-Amino-4-Trifluoromethylcoumarin (MP Biomedicals, Santa Ana, CA) for 5 minutes using a Synergy MX microplate reader (Biotek, Winooski, VT) and expressed as fluorescence intensity or ∆ fluorescence intensity per minute.
Statistical analyses
Data were presented as mean ± standard error of the mean (SEM). GraphPad Prism 7 software was used for statistical analyses. Differences between groups were analyzed using analysis of variance followed by Tukey test. A P < .05 is considered statistically significant.
Results
tPA-mediated PKal activation via plasmin and FXII in human plasma
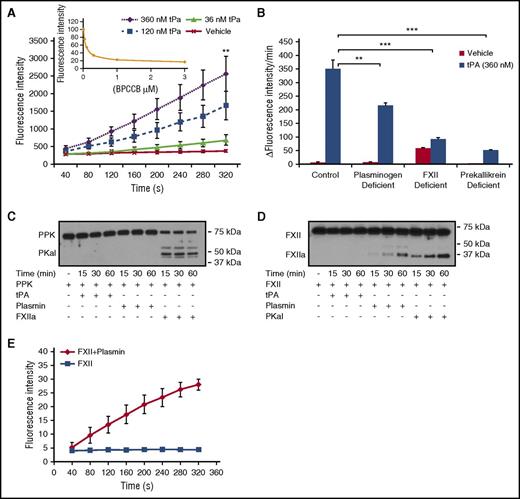
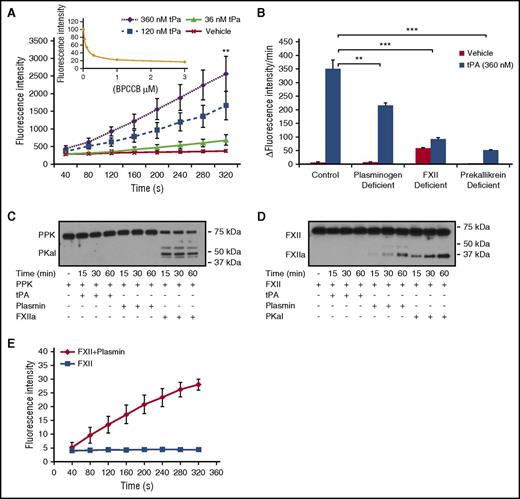
To examine the effect of tPA on PKal formation in human plasma, purified human tPA (0 to 360 nM) was incubated with plasma for 10 minutes, and kallikrein-like activity was measured as the rate of hydrolysis of a PKal substrate (D-Pro-Phe-Arg-7-Amino-4-Trifluoromethylcoumarin). tPA induced concentration-dependent increases in kallikrein-like activity (P < .01 vs vehicle; Figure 1A). The contribution of PKal to tPA-stimulated kallikrein activity was demonstrated using a selective PKal inhibitor BPCCB25,26 (Figure 1A, insert) and PPK-deficient plasma (Figure 1B), which decreased substrate hydrolysis by 83% and 74%, respectively. To investigate the factors that may contribute to tPA-mediated PKal activity, we incubated tPA (360 nM) with normal human plasma and with plasma-depleted in plasminogen or FXII. We show that plasminogen deficiency and FXII deficiency decreased tPA-induced kallikrein-like-activity by ∼38% (P < .01) and 74% (P < .001), respectively, compared with kallikrein-like activity in control plasma incubated with tPA (Figure 1B). We next examined whether the modulation of KKS by tPA may involve a direct interaction between tPA or plasmin with PPK or FXII in a purified protein assay. Incubation of purified human PPK with FXIIa reduced levels of PPK and generated a band at 50 kDa, which is the predicted size of PKal heavy chain (Figure 1C). In contrast, PPK cleavage was not observed following incubations with either plasmin or tPA. Incubation of purified human FXII with plasmin and PKal generated fragments at 30 and 29 kDa, respectively (Figure 1D). In contrast, tPA did not appear to generate lower-molecular-weight fragments of FXII. In addition, we found that incubation of purified FXII with plasmin resulted in increased FXIIa activity (Figure 1E). We next evaluated whether PKal plays a role in clot formation and lysis induced by thrombin and tissue factor. Turbidimetric analysis of human plasma incubated with BPCCB (1 and 3 μM) showed that PKal inhibition decreased clot formation rate induced by thrombin (1330 ± 35 seconds vehicle vs 1633 ± 71 seconds BPCCB [3 μM], P < .05; supplemental Figure 1A-B), whereas in combination with tPA, BPCCB accelerated the rate of clot lysis when compared with tPA alone (2302 ± 180 seconds vehicle vs 1703 ± 111 seconds BPCCB [3 μM], P < .05; supplemental Figure 1C-D). Moreover, we show that PPK-deficient human plasma displayed delayed clot formation induced by thrombin compared with normal plasma (1330 ± 35 seconds vehicle vs 2240 ± 141 seconds PPK-deficient, P < .01; supplemental Figure 1A-B). In the tPA-induced clot lysis assay, PPK-deficient plasma displayed a decreased magnitude of turbidity compare with normal plasma, suggesting reduced clot formation (supplemental Figure 1C-D). In contrast, PKal inhibition and PPK deficiency did not alter clot formation and lysis induced by tissue factor (supplemental Figure 1E-H).
PKal is activated by tPA in human plasma. (A) Concentration-response curves of tPA in human plasma. Purified human tPA (0-360 nM) was incubated in human plasma for 10 minutes at room temperature. Kallikrein-like activity was determined by measuring changes in fluorescence at 410 nm because of hydrolysis of D-Pro-Phe-Arg-7-Amino-4-Trifluoromethylcoumarin. Data are expressed as fluorescence intensity and represent mean ± SEM of 3 independent experiments. **P < .01 vs vehicle. Insert: Dose response of inhibition of kallikrein-like activity by BPCCB (0.1-3 μM; n = 3 independent experiments). (B) Effects of tPA on kallikrein-like activity in normal, plasminogen-, FXII-, or prekallikrein-deficient plasma. Data are expressed as ∆ fluorescence intensity per minute and represent mean ± SEM of 3 independent experiments. **P < .01; ***P < .001. (C) Time course of PKal production by FXIIa. Purified human PPK (0.4 μM) was incubated with 0.5 μM purified human tPA or 0.5 μM purified human plasmin or 0.5 μM purified human FXIIa for time course. Analysis of the incubation mixture by western blot demonstrates PPK cleavage by FXIIa. (D) Time course of FXIIa production by plasmin and PKal. Purified human FXII (0.37 μM) was incubated with 0.5 μM purified human tPA or 0.5 μM purified human plasmin or 0.5 μM purified human PKal for time course. Analysis of the incubation mixture by western blot demonstrates FXII cleavage by plasmin and PKal. (E) Time course of FXIIa activity. Data are expressed as fluorescence intensity and represent mean ± SEM of 3 independent experiments.
PKal is activated by tPA in human plasma. (A) Concentration-response curves of tPA in human plasma. Purified human tPA (0-360 nM) was incubated in human plasma for 10 minutes at room temperature. Kallikrein-like activity was determined by measuring changes in fluorescence at 410 nm because of hydrolysis of D-Pro-Phe-Arg-7-Amino-4-Trifluoromethylcoumarin. Data are expressed as fluorescence intensity and represent mean ± SEM of 3 independent experiments. **P < .01 vs vehicle. Insert: Dose response of inhibition of kallikrein-like activity by BPCCB (0.1-3 μM; n = 3 independent experiments). (B) Effects of tPA on kallikrein-like activity in normal, plasminogen-, FXII-, or prekallikrein-deficient plasma. Data are expressed as ∆ fluorescence intensity per minute and represent mean ± SEM of 3 independent experiments. **P < .01; ***P < .001. (C) Time course of PKal production by FXIIa. Purified human PPK (0.4 μM) was incubated with 0.5 μM purified human tPA or 0.5 μM purified human plasmin or 0.5 μM purified human FXIIa for time course. Analysis of the incubation mixture by western blot demonstrates PPK cleavage by FXIIa. (D) Time course of FXIIa production by plasmin and PKal. Purified human FXII (0.37 μM) was incubated with 0.5 μM purified human tPA or 0.5 μM purified human plasmin or 0.5 μM purified human PKal for time course. Analysis of the incubation mixture by western blot demonstrates FXII cleavage by plasmin and PKal. (E) Time course of FXIIa activity. Data are expressed as fluorescence intensity and represent mean ± SEM of 3 independent experiments.
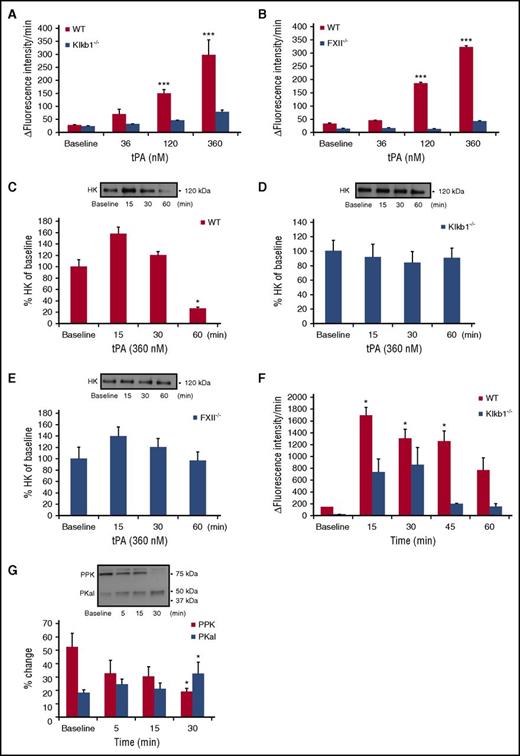
Effect of tPA on PKal activity in mouse plasma
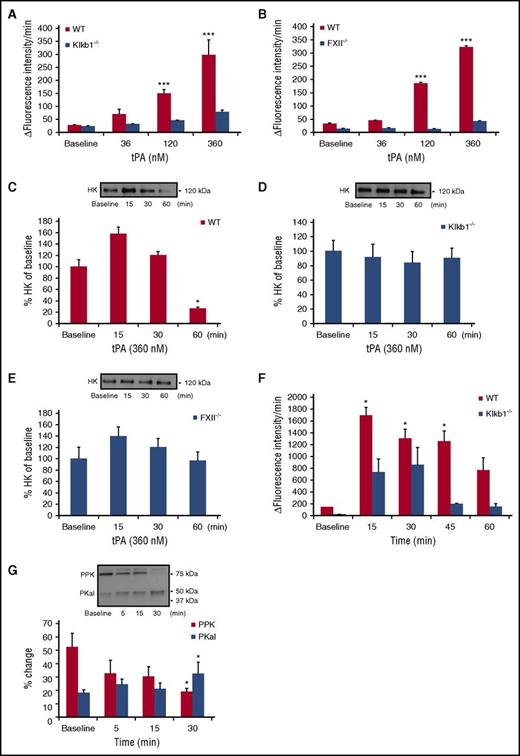
Next, we examined the effect of tPA on kallikrein-like activity in mouse plasma. Incubation of WT mouse plasma with tPA induced concentration-dependent increases in kallikrein-like activity (P < .001 vs WT; Figure 2A). Incubation of tPA with plasma from PPK-deficient mice (Klkb1−/−) showed markedly reduced kallikrein-like activity compared with WT plasma, confirming that hydrolysis of the substrate was mainly attributable to PKal (Figure 2A). Incubation of tPA with plasma from FXII-deficient (FXII−/−) mice displayed markedly reduced kallikrein-like activity compared with plasma form WT mice (P < .001; Figure 2B). Because tPA is associated with increased bradykinin from HK cleavage,31 we examine whether this cleavage required PPK and FXII. Cleavage of endogenous HK was measured in plasma samples from WT, Klkb1−/−, and FXII−/− mice in the presence or absence of tPA (360 nM). We show that tPA increased HK cleavage at 60 minutes in WT plasma (P < .05; Figure 2C). In contrast, tPA did not induce HK cleavage in plasma from either Klkb1−/− or FXII−/− mice (Figure 2D-E). Next, we explored the effects of intravenous injection of tPA in mice on PKal activity in WT and Klkb1−/− mice. tPA was administered as a bolus (10 mg/kg) via tail vein and blood was collected at 5, 15, 30, 45, and 60 minutes after injection. We found that tPA rapidly increased kallikrein-like activity at 15 minutes (P < .05), which was sustained for at least 1 hour postinjection (Figure 2F). A transient increase in amidolytic activity was also observed in Klkb1−/− mice but to a lesser extent compared with WT mice. Western blot analysis of plasma from WT mice receiving tPA injection showed a decrease in PPK (P < .05; 75 kDa) and a concomitant increase in the PKal heavy chain 50 kDa (P < .05; Figure 2G).
Effects of tPA on PKal activity in mouse plasma. (A) Concentration-response curves of tPA in WT and Klkb1−/− mice plasma. Purified human tPA (0-360 nM) was incubated in mouse plasma in vitro for 10 minutes at room temperature. Kallikrein-like activity was measured as fluorescence at 410 nm because of hydrolysis of D-Pro-Phe-Arg-7-Amino-4-Trifluoromethylcoumarin. Data are expressed as ∆ fluorescence intensity per minute and represent mean ± SEM of 3 independent experiments. ***P < .001 vs baseline. (B) Concentration-response curves of tPA in WT and FXII−/− mice plasma. Purified human tPA (0-360 nM) was incubated in mouse plasma in vitro for 10 minutes at room temperature. Data are expressed as ∆ fluorescence intensity per minute and represent mean + SEM of 3 independent experiments. ***P < .001 vs baseline. (C) Bar graph displays the time course of HK in WT mouse plasma incubated with tPA (360 nM). Representative western blot image is shown on top of the bar graph. Data are presented as mean ± SEM, n = 4. *P < .05 vs baseline. (D) Bar graph displays the time course of HK in Klkb1−/− mouse plasma incubated with tPA (360 nM). Representative western blot image is shown on top of the bar graph. Data are presented as mean ± SEM, n = 4. (E) Bar graph displays the time course of HK in FXII−/− mouse plasma incubated with tPA (360 nM). Representative western blot image is shown on top of the bar graph. Data are presented as mean ± SEM, n = 4. (F) Time-response of PPK activation in mouse plasma after tPA injection. Mice were treated intravenously with tPA (10 mg/kg) and blood collected at 15, 30, 45, and 60 minutes after injection. Data are expressed as ∆ fluorescence intensity per minute and represent mean ± SEM of 4 independent experiments. *P < .05 vs baseline. (G) Bar graph displays the time course of PKal generation after tPA injection. Representative western blot image is shown on top of the bar graph. Data are presented as mean ± SEM, n = 4. *P < .05 vs baseline.
Effects of tPA on PKal activity in mouse plasma. (A) Concentration-response curves of tPA in WT and Klkb1−/− mice plasma. Purified human tPA (0-360 nM) was incubated in mouse plasma in vitro for 10 minutes at room temperature. Kallikrein-like activity was measured as fluorescence at 410 nm because of hydrolysis of D-Pro-Phe-Arg-7-Amino-4-Trifluoromethylcoumarin. Data are expressed as ∆ fluorescence intensity per minute and represent mean ± SEM of 3 independent experiments. ***P < .001 vs baseline. (B) Concentration-response curves of tPA in WT and FXII−/− mice plasma. Purified human tPA (0-360 nM) was incubated in mouse plasma in vitro for 10 minutes at room temperature. Data are expressed as ∆ fluorescence intensity per minute and represent mean + SEM of 3 independent experiments. ***P < .001 vs baseline. (C) Bar graph displays the time course of HK in WT mouse plasma incubated with tPA (360 nM). Representative western blot image is shown on top of the bar graph. Data are presented as mean ± SEM, n = 4. *P < .05 vs baseline. (D) Bar graph displays the time course of HK in Klkb1−/− mouse plasma incubated with tPA (360 nM). Representative western blot image is shown on top of the bar graph. Data are presented as mean ± SEM, n = 4. (E) Bar graph displays the time course of HK in FXII−/− mouse plasma incubated with tPA (360 nM). Representative western blot image is shown on top of the bar graph. Data are presented as mean ± SEM, n = 4. (F) Time-response of PPK activation in mouse plasma after tPA injection. Mice were treated intravenously with tPA (10 mg/kg) and blood collected at 15, 30, 45, and 60 minutes after injection. Data are expressed as ∆ fluorescence intensity per minute and represent mean ± SEM of 4 independent experiments. *P < .05 vs baseline. (G) Bar graph displays the time course of PKal generation after tPA injection. Representative western blot image is shown on top of the bar graph. Data are presented as mean ± SEM, n = 4. *P < .05 vs baseline.
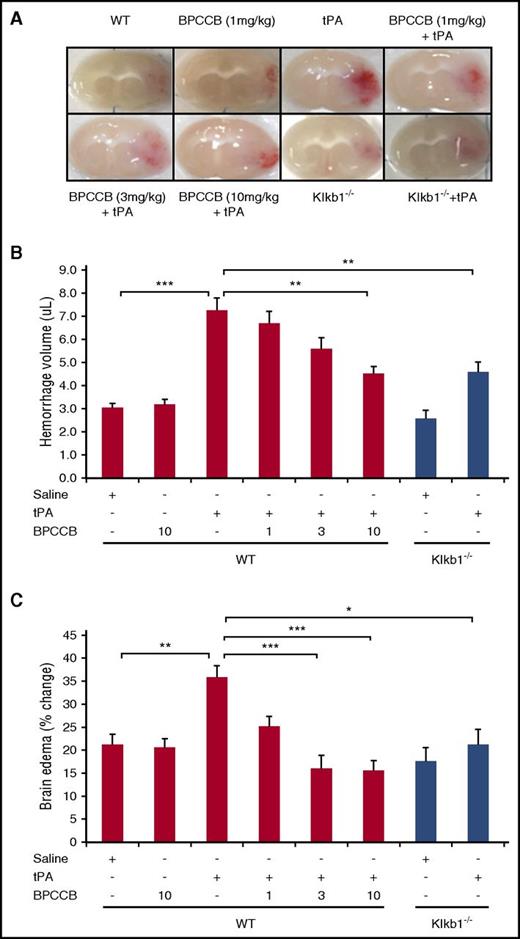
PKal inhibition reduces tPA-mediated brain hemorrhage after stroke in mice
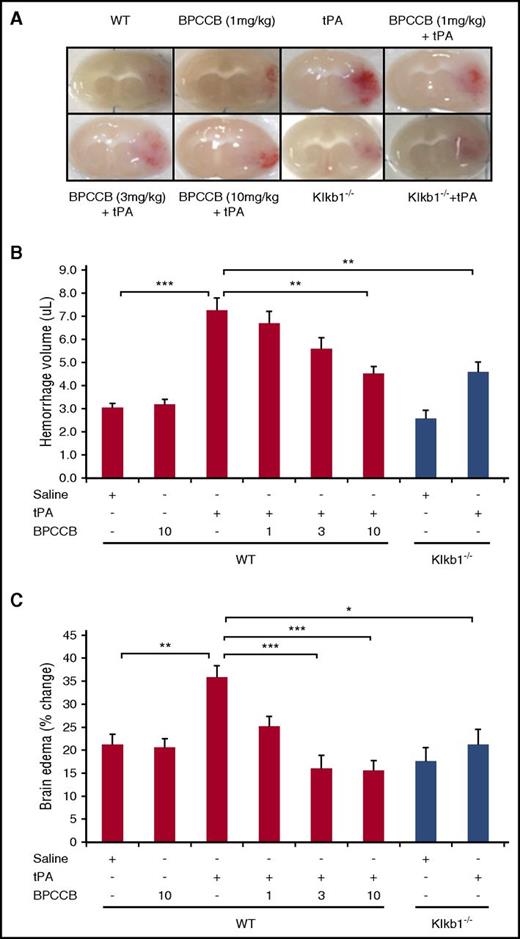
To examine whether the combination of PKal inhibitor with tPA influences the incidence of hemorrhagic transformation, we evaluated hemorrhage volume at 24 hours after ischemia. Infusion of tPA (10 mg/kg) in mice subjected to stroke resulted in increased brain hemorrhage when compared with saline-treated mice (3.0 ± 0.2 μL WT vs 7.3 ± 0.5 μL WT + TPA, P < .001; Figure 3A-B). Coadministration of BPCCB with tPA after stroke resulted in reduced hemorrhage volume in a concentration-dependent manner (7.3 ± 0.5 μL WT + tPA vs 6.7 ± 0.5 μL WT + BPCCB [1 mg/kg] + tPA, not significant [NS]; 5.6 ± 0.5 μL WT + BPCCB [3 mg/kg] + tPA, NS; 4.5 ± 0.3 μL WT + BPCCB [10 mg/kg] + tPA, P < .01; Figure 3A-B). Moreover, the effect of PKal on hemorrhagic transformation induced by tPA was also evaluated in Klkb1−/− mice. Hemorrhage volume was smaller in tPA-treated Klkb1−/− mice compared with tPA-treated WT mice (4.6 ± 0.4 μL Klkb1−/− vs 7.3 ± 0.5 μL WT + tPA, P < .01; Figure 3A-B). Coadministration of BPCCB with tPA reduced brain edema in a concentration-dependent manner when compared with tPA alone (35% WT + tPA vs 25% WT + BPCCB [1 mg/kg] + tPA, NS; 16% WT + BPCCB [3 mg/kg] + tPA, P < .001; 15% WT + BPCCB [10 mg/kg] + tPA, P < .001; Figure 3C). The ischemic hemisphere of WT mice treated with tPA showed significantly increased brain edema compared with tPA-treated Klkb1−/− mice (P < .01 vs WT ± tPA; Figure 3C).
Effect of PKal on tPA-mediated intracerebral hemorrhage transformation after ischemic stroke in mice. (A) Representative coronal sections of brain mice at 24 hours after stroke. Red color indicate hemoglobin associated with hemorrhage transformation within the ischemic area in mice treated with tPA and the decreased hemorrhage associated with PKal inhibition on tPA-induced postischemic brain. At 2 hours after stroke onset, mice received tPA (10 mg/kg) either alone of following a 15 minutes pretreatment with BPCCB (1, 3, 10 mg/kg) or saline vehicle. Klkb1−/− mice were treated intravenously with saline or tPA (10 mg/kg). (B) Volumes of intracerebral hemorrhage were quantified with hemoglobin assay at 24 hours after stroke. Data are presented as mean ± SEM, n = 6-8. **P < .01; ***P < .001. (C) Quantitative analysis of brain edema at 24 hours after ischemic stroke. Data are presented as mean ± SEM, n = 7-8. *P < .05; **P < .01; ***P < .001. Numbers in x-axis labels for panels B and C indicate concentrations of BPCCB in milligrams per kilogram.
Effect of PKal on tPA-mediated intracerebral hemorrhage transformation after ischemic stroke in mice. (A) Representative coronal sections of brain mice at 24 hours after stroke. Red color indicate hemoglobin associated with hemorrhage transformation within the ischemic area in mice treated with tPA and the decreased hemorrhage associated with PKal inhibition on tPA-induced postischemic brain. At 2 hours after stroke onset, mice received tPA (10 mg/kg) either alone of following a 15 minutes pretreatment with BPCCB (1, 3, 10 mg/kg) or saline vehicle. Klkb1−/− mice were treated intravenously with saline or tPA (10 mg/kg). (B) Volumes of intracerebral hemorrhage were quantified with hemoglobin assay at 24 hours after stroke. Data are presented as mean ± SEM, n = 6-8. **P < .01; ***P < .001. (C) Quantitative analysis of brain edema at 24 hours after ischemic stroke. Data are presented as mean ± SEM, n = 7-8. *P < .05; **P < .01; ***P < .001. Numbers in x-axis labels for panels B and C indicate concentrations of BPCCB in milligrams per kilogram.
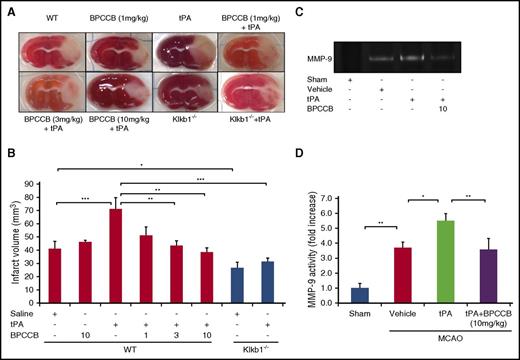
Effects of PKal on brain injury from thrombotic stroke in mice treated with tPA
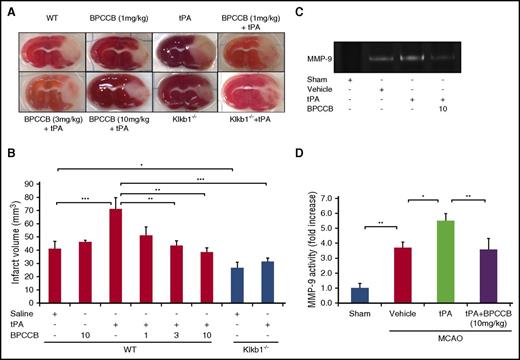
To examine the role of PKal after thrombolytic therapy with tPA in acute ischemic stroke, we evaluated the effects of PKal inhibition on infarct volume in ischemic brain by staining brain sections with triphenyltetrazolium chloride (Figure 4A) 24 hours after MCAO. Infarct volume at 24 hours after stroke in mice infused with tPA after MCAO was increased compared with saline (vehicle)–infused mice at 24 hours after stroke (41.3 ± 5.4 mm3 WT vs 71.3 ± 8.3 mm3 WT + tPA; P < .001), and coadministration of BPCCB with tPA reduced infarct volume in a concentration-dependent manner (71.3 ± 8.3 mm3 WT + tPA vs 51.4 ± 6.1 mm3 WT + BPCCB [1 mg/kg] + tPA, NS; 43.5 ± 3.6 mm3 WT + BPCCB [3 mg/kg] + tPA, P < .01; 38.5 ± 3.2 mm3 WT + BPCCB [10 mg/kg] + tPA, P < .01; Figure 4B). To further evaluate the effects of KKS on tPA’s effects, we used Klkb1−/− mice. Infarct volumes in untreated mice were reduced by 32% in Klkb1−/− mice compared with responses in WT mice (41.3.1 ± 5.4 mm3 WT vs 26.7 ± 4.4 mm3 Klkb1−/−, P < .05; Figure 4B). tPA-treated Klkb1−/− mice showed a smaller infarct volume when compared with tPA-treated WT mice (31.5 ± 2.5 mm3 Klkb1−/− + tPA vs 71.3 ± 8.3 mm3 WT + tPA; P < .001; Figure 4B).
PKal inhibition reduces tPA-induced increases in infarct volume and MMP-9 activation after stroke. (A) Representative coronal sections of 2,3,5-triphenyltetrazolium chloride–stained brain sections of mice at 24 hours after ischemic stroke. At 2 hours after stroke onset, mice received tPA (10 mg/kg) either alone of following a 15 minutes pretreatment with BPCCB (1, 3, 10 mg/kg) or saline vehicle. Klkb1−/− mice were treated intravenously with saline or tPA (10 mg/kg). Ischemic infarctions (white color area) were detected in all groups; however, tPA alone showed a remarkably ischemic volume. (B) Quantitative analysis of infarct volume at 24 hours after ischemic stroke. *P < .05; **P < .01; ***P < .001. Data are presented as mean ± SEM, n = 5-8. Numbers in x-axis labels for panel B indicate concentrations of BPCCB in mg/kg. (C) Coadministration of PKal inhibitor BPCCB with tPA decreases MMP-9 activation in brain after stroke. Representative zymographic analysis of brain extracts from sham and stroke mice treated with vehicle, tPA (10 mg/kg), or BPCCB (10 mg/kg) after MCAO and analyzed at 24 hours after stroke. (D) Quantitative determination of zymographic gels for each treatment. Data are presented as mean ± SEM, n = 5. *P < .05; **P < .01.
PKal inhibition reduces tPA-induced increases in infarct volume and MMP-9 activation after stroke. (A) Representative coronal sections of 2,3,5-triphenyltetrazolium chloride–stained brain sections of mice at 24 hours after ischemic stroke. At 2 hours after stroke onset, mice received tPA (10 mg/kg) either alone of following a 15 minutes pretreatment with BPCCB (1, 3, 10 mg/kg) or saline vehicle. Klkb1−/− mice were treated intravenously with saline or tPA (10 mg/kg). Ischemic infarctions (white color area) were detected in all groups; however, tPA alone showed a remarkably ischemic volume. (B) Quantitative analysis of infarct volume at 24 hours after ischemic stroke. *P < .05; **P < .01; ***P < .001. Data are presented as mean ± SEM, n = 5-8. Numbers in x-axis labels for panel B indicate concentrations of BPCCB in mg/kg. (C) Coadministration of PKal inhibitor BPCCB with tPA decreases MMP-9 activation in brain after stroke. Representative zymographic analysis of brain extracts from sham and stroke mice treated with vehicle, tPA (10 mg/kg), or BPCCB (10 mg/kg) after MCAO and analyzed at 24 hours after stroke. (D) Quantitative determination of zymographic gels for each treatment. Data are presented as mean ± SEM, n = 5. *P < .05; **P < .01.
Coadministration of a PKal inhibitor with tPA decreases MMP-9 activation in brain after stroke
Previous reports have shown that matrix metalloproteinase-9 (MMP-9) contributes to hemorrhage in MCAO mice treated with tPA.27 Because PKal inhibition reduced tPA-induced brain hemorrhage, we investigated whether PKal contributes to MMP-9 activation. Gelatin-zymography of brain tissue indicated a marked increase in MMP-9 in mice subjected to MCAO compared with MMP-9 levels in sham mice (P < .01; Figure 4C-D). We observe increased MMP-9 activity in tPA-treated mice after stroke compared with mice with stroke receiving saline (P < .05 vs vehicle), and coadministration of BPCCB (10 mg/kg) with tPA reduced MMP-9 activity induced by tPA (P < .01 vs tPA; Figure 4C-D).
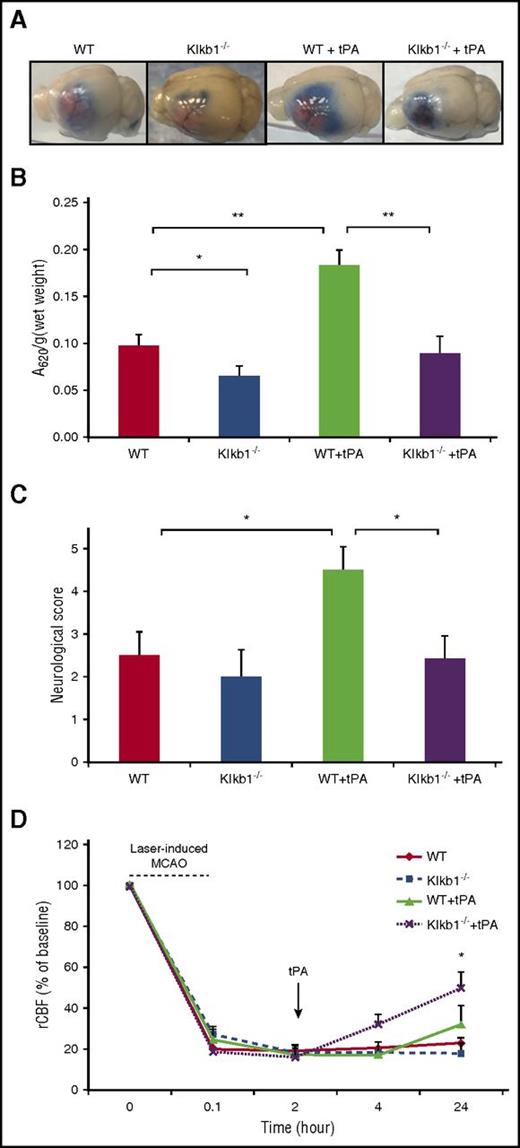
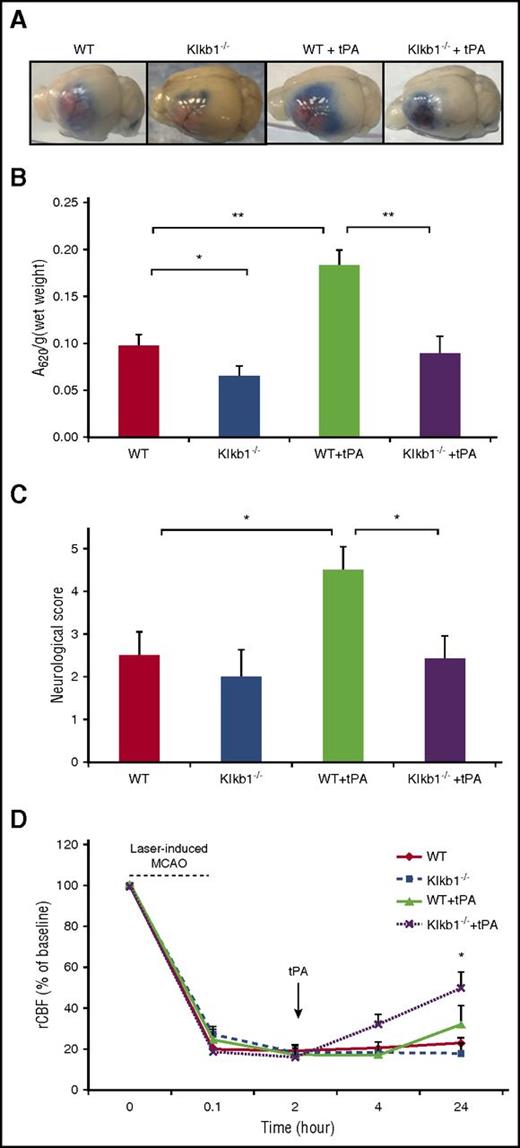
PKal mediates tPA-induced blood-brain barrier (BBB) leakage
To examine whether PKal contributes to tPA-induced BBB dysfunction during stroke, we examined cerebral vascular permeability in WT and Klkb1−/− mice subjected to MCAO and tPA with Evans blue dye (Figure 5A). Mice with PPK deficiency treated with tPA showed a 54% reduction in Evans blue dye extravasation after MCAO compared with tPA-treated WT mice (0.098 ± 0.012 Klkb1−/− + tPA vs 0.18 ± 0.02 WT + tPA, P < .01; Figure 5B), suggesting that KKS contributes to tPA-induced cerebrovascular permeability after stroke. In the absence of tPA, MCAO-induced Evans blue dye extravasation was reduced by 33%, in Klkb1−/− mice compared with WT mice (0.098 ± 0.01 WT vs 0.065 ± 0.0.1 Klkb1−/−, P < .05; Figure 5B). Furthermore, tPA-treated WT mice with MCAO showed more severe neurological deficits compared with mice subjected to MCAO receiving saline (P < .05 vs WT). In addition, the neurological scores were significantly better in the tPA-treated Klkb1−/− mice with MCAO compared with tPA-treated WT mice with MCAO (P < .05 vs WT + tPA; Figure 5C). We also evaluated the effect of PPK deficiency on tPA-induced brain hemisphere reperfusion in mice with photothrombotic MCAO. Regional cerebral blood flow (rCBF) was measured using a laser Doppler flowmeter for up to 2 hours after tPA treatment and later at 4 and 24 hours after stroke. PPK deficiency increased tPA-mediated reperfusion in mice at 24 hours after stroke when compared with tPA-treated alone (49% Klkb1−/− + tPA vs 30% WT + tPA, P < .05; Figure 5D).
PPK deficiency reduced tPA-induced BBB leakage after ischemic stroke. (A) Representative images of Evans blue extravasations from WT and Klkb1−/− treated with saline or tPA (10 mg/kg) and euthanized at 24 hours after stroke. (B) Quantification of Evans blue fluorescent intensity for each group. Data are presented as mean ± SEM, n = 5-6. *P < .05; **P < .01. (C) Effects of PKal inhibition on tPA-induced neurological dysfunction after ischemic stroke. Neurological severity score was evaluated 24 hours after stroke in WT and Klkb1−/− mice treated with saline or tPA (10 mg/kg). Data are presented as mean ± SEM, n = 5-7. *P < .05. (D) PKal inhibition accelerates reperfusion during tPA treatment after stroke. Quantitative analysis of rCBF showed facilitated reperfusion in Klkb1−/− mice. Laser-Doppler flowmeter was used to monitor rCBF for up to 2 hours and at 24 hours after tPA treatment. Data are presented as mean ± SEM, n = 3-6. *P < .05 vs WT + tPA.
PPK deficiency reduced tPA-induced BBB leakage after ischemic stroke. (A) Representative images of Evans blue extravasations from WT and Klkb1−/− treated with saline or tPA (10 mg/kg) and euthanized at 24 hours after stroke. (B) Quantification of Evans blue fluorescent intensity for each group. Data are presented as mean ± SEM, n = 5-6. *P < .05; **P < .01. (C) Effects of PKal inhibition on tPA-induced neurological dysfunction after ischemic stroke. Neurological severity score was evaluated 24 hours after stroke in WT and Klkb1−/− mice treated with saline or tPA (10 mg/kg). Data are presented as mean ± SEM, n = 5-7. *P < .05. (D) PKal inhibition accelerates reperfusion during tPA treatment after stroke. Quantitative analysis of rCBF showed facilitated reperfusion in Klkb1−/− mice. Laser-Doppler flowmeter was used to monitor rCBF for up to 2 hours and at 24 hours after tPA treatment. Data are presented as mean ± SEM, n = 3-6. *P < .05 vs WT + tPA.
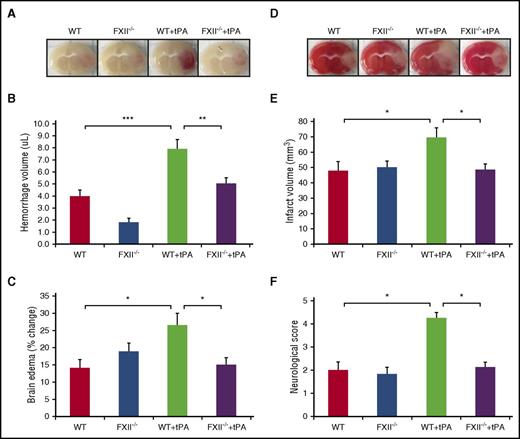
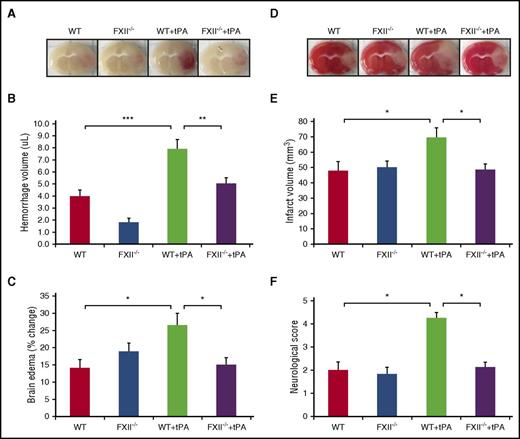
FXII deficiency reduces infarct volume and edema after tPA treatment in thrombotic stroke without increasing the risk of intracerebral hemorrhage
Because tPA increases FXII activation through plasmin and results in PPK activation, we evaluated whether FXII-deficient mice are protected against brain injury induced by tPA treatment after MCAO. Images of coronal sections show hemorrhage transformation 24 hours after stroke in tPA-treated WT mice compared with tPA-treated FXII−/− mice (Figure 6A). The effect of FXII on hemorrhagic transformation induced by tPA was smaller in tPA-treated FXII−/− mice compared with tPA-treated WT mice (5.1 + 0.4 μL Klkb1−/− vs 7.9 + 0.8 μL WT + tPA, P < .01; Figure 6B). The ischemic hemisphere of WT mice treated with tPA showed significantly increased brain edema compared with tPA-treated FXII−/− mice (P < .05 vs WT + tPA; Figure 6C). Furthermore, tPA-treated FXII−/− mice showed a smaller infarct volume when compared with tPA-treated WT mice (48.4 + 3.8 mm3 Klkb1−/− + tPA vs 69.4 + 6.4 mm3 WT + tPA, P < .05; Figure 6D-E) 24 hours after stroke. In addition, tPA-treated WT mice with MCAO showed more severe neurological deficits compared with mice subjected to MCAO receiving saline (P < .05 vs WT). The neurological scores were significantly better in the tPA-treated FXII−/− mice with MCAO compared with tPA-treated WT mice with MCAO (P < .05 vs WT + tPA; Figure 6F).
FXII deficiency reduces infarct volume and edema after tPA treatment in thrombotic stroke without increasing the risk of intracerebral hemorrhage. (A) Representative images of coronal sections of show cerebral hemorrhage 24 hours after stroke in mice treated with vehicle or tPA in WT and FXII−/− mice. At 2 hours after stroke onset, mice received tPA (10 mg/kg) or saline vehicle. (B) Volumes of intracerebral hemorrhage were quantified with hemoglobin assay at 24 hours after stroke. Data are presented as mean ± SEM, n = 8. **P < .01; ***P < .001. (C) Quantitative analysis of brain edema at 24 hours after ischemic stroke. Data are presented as mean ± SEM, n = 8. *P < .05. (D) Representative coronal sections of 2,3,5-triphenyltetrazolium chloride–stained brain sections of mice at 24 hours after ischemic stroke treated with vehicle or tPA in WT and FXII−/− mice. (E) Quantitative analysis of infarct volume at 24 hours after ischemic stroke. Data are presented as mean ± SEM, n = 8. *P < .05. (F) Effects of FXII inhibition on tPA-induced neurological dysfunction after ischemic stroke. Neurological severity score was evaluated 24 hours after stroke in WT and FXII−/− mice treated with saline or tPA (10 mg/kg). Data are presented as mean ± SEM, n = 5-8. *P < .05.
FXII deficiency reduces infarct volume and edema after tPA treatment in thrombotic stroke without increasing the risk of intracerebral hemorrhage. (A) Representative images of coronal sections of show cerebral hemorrhage 24 hours after stroke in mice treated with vehicle or tPA in WT and FXII−/− mice. At 2 hours after stroke onset, mice received tPA (10 mg/kg) or saline vehicle. (B) Volumes of intracerebral hemorrhage were quantified with hemoglobin assay at 24 hours after stroke. Data are presented as mean ± SEM, n = 8. **P < .01; ***P < .001. (C) Quantitative analysis of brain edema at 24 hours after ischemic stroke. Data are presented as mean ± SEM, n = 8. *P < .05. (D) Representative coronal sections of 2,3,5-triphenyltetrazolium chloride–stained brain sections of mice at 24 hours after ischemic stroke treated with vehicle or tPA in WT and FXII−/− mice. (E) Quantitative analysis of infarct volume at 24 hours after ischemic stroke. Data are presented as mean ± SEM, n = 8. *P < .05. (F) Effects of FXII inhibition on tPA-induced neurological dysfunction after ischemic stroke. Neurological severity score was evaluated 24 hours after stroke in WT and FXII−/− mice treated with saline or tPA (10 mg/kg). Data are presented as mean ± SEM, n = 5-8. *P < .05.
Discussion
This report identifies the contact system as a mediator of brain injury induced by intravenous administration of tPA in mice with thrombotic MCAO. We show that pharmacological inhibition of PKal or PPK and FXII deficiency in mice reduces intracerebral hemorrhage, edema, and infarct volume induced by tPA. The beneficial effects of PPK deficiency are associated with increased tPA-induced cerebral blood flow. These findings suggest that coadministration of a PKal or a FXIIa inhibitor with tPA may provide an opportunity to reduce cerebral complications and improve efficacy of thrombolytic therapy for ischemic stroke.
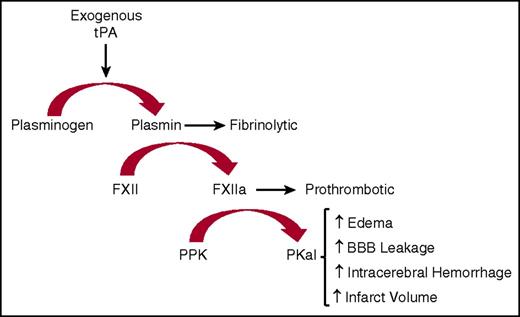
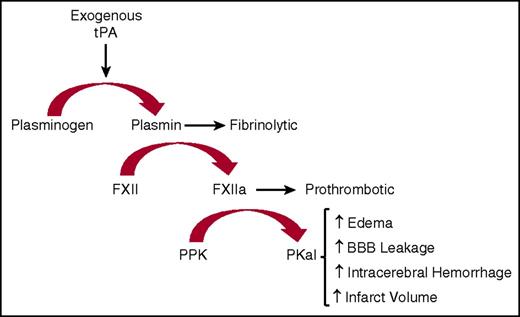
We show that intravenous infusion of tPA in mice increases circulating kallikrein-like activity. In vitro studies using either PPK-deficient human or mouse plasma revealed that most, but not all, of this amidolytic activity is associated with PKal, suggesting that tPA increases the activity of additional plasma proteases detected by this assay. Consistent with these findings, we show that both plasminogen and FXII contribute to tPA-stimulated kallikrein-like activity in plasma. Studies using purified proteins revealed that plasmin cleaves FXII into a fragment with a molecular weight at or slightly higher than the expected size of FXIIa light chain. We did not observe plasmin-mediated cleavage of PPK, and tPA did not appear to cleave FXII or PPK, suggesting that tPA-stimulated kallikrein-like activity is mediated, at least in part, by plasmin’s effects on FXII. These findings are consistent with previous reports showing that incubation of FXII with plasmin, both as purified enzymes and in plasma, increases FXIIa activity.9,24,32,33 Plasmin’s effects on purified FXIIa activity is markedly increased in the presence of dextran sulfate and inhibited by e-aminocaprioic acid and FXIIa inhibitory mAb.24 These reports have suggested that plasmin-mediated cleavage of FXII increases FXIIa-like activity; however, the mechanisms that mediate the effects of plasmin on FXIIa activity are not fully understood. A previous study using N-terminal sequencing revealed FXII cleavage at both Arg353 and Lys346 following incubation with plasmin.9 Cleavage of FXII at Arg353 leads to its zymogen activation to FXIIa.9 Although the consequences of plasmin-mediated cleavage of FXII at Lys346 on FXIIa-like catalytic activity are not yet available, this cleavage may cause a conformational change that facilitates its effects as a KKS activator. This can be seen with dextran sulfate in the action of FXII without a concomitant clotting reaction.34,35 tPA alone appears to be sufficient to trigger FXII activity and the contact system without surface binding or polymeric negative charge. Therefore, generation of plasmin through tPA treatment proteolytically activates FXII and thereby leads to activation of the KKS cascade (Figure 7).
Schematic diagram illustrating the actions of exogenous tPA on KKS after stroke. Exogenous tPA activates plasminogen to plasmin that then activates FXII to FXIIa. Formed FXIIa activates PPK to PKal. Formed PKal contributes to stroke progression by increasing edema, intracerebral hemorrhage, BBB leakage, and infarct volume.
Schematic diagram illustrating the actions of exogenous tPA on KKS after stroke. Exogenous tPA activates plasminogen to plasmin that then activates FXII to FXIIa. Formed FXIIa activates PPK to PKal. Formed PKal contributes to stroke progression by increasing edema, intracerebral hemorrhage, BBB leakage, and infarct volume.
Our results showing that tPA increases kallikrein-like activity in mice in vivo are consistent with clinical findings showing that infusion of alteplase in patients with an acute myocardial infarction increases kallikrein activity in plasma.36 Systemic activation of the KKS by tPA could have both peripheral and cerebral effects. Peripheral activation of PKal by tPA could contribute to orolingual angioedema that has been reported to occur at an incidence of up to 5% of cases during thrombolytic therapy and has been attributed to bradykinin.18-20 Recently, Marcos-Contreras et al demonstrated that tPA infusion after acute stroke in humans triggers HK cleavage and bradykinin generation.31 In addition, this report showed that plasmin can cleave HK in a purified system. Our results demonstrate that HK cleavage by tPA in plasma requires PPK and FXII, suggesting that tPA-induced cleavage of HK is mediated by a cascade of plasmin, FXIIa, and PKal activities. Thus, inhibition of either PKal or FXIIa may provide opportunities to block off target effects of tPA on bradykinin production. Regarding the physiological consequences of this pathway, the main finding in this report is that PKal activity contributes to cerebral complications associated with tPA therapy in a murine model of ischemic stroke. We show that PKal inhibition and PPK deficiency reduce intracerebral hemorrhage, edema, and infarct volume caused by intravenous administration of tPA after thrombotic MCAO. The protective effects against brain infarct volume seen in Klkb1-deficient mice are consistent with previous findings showing that PPK deficiency reduces brain injury caused by permanent MCAO.15 On the other hand, treatment with a Pkal inhibitor after permanent MCAO did not show any neuroprotective effect as demonstrated by Storini et al.37 The mechanisms that contribute to this neuroprotection by PKal blockade are not fully understood and could involve reduced inflammation and improved vascular perfusion. In addition, intravenous administration of tPA into FXII-deficient mice after stroke reduces intracerebral hemorrhage, edema, and infarct volume. These findings support the concept that activated FXII activates PKal during tPA treatment in WT mice after stroke. In agreement with Pham et al,38 FXII-deficient mice were not protected against infarction in the permanent MCAO model in the absence of exogenous tPA in our study. We also show that PKal blockade reduced edema and cerebrovascular permeability induced by tPA. This is consistent with the well-documented effects of the KKS in promoting vascular permeability and vasogenic edema. Most of the edematous effects of the KKS have been attributed to bradykinin and its metabolite desArg9 -bradykinin, which activate their G protein–coupled receptors, B2R and B1R, respectively, and thereby increase vascular permeability.39 Although the therapeutic benefits of tPA have been demonstrated for up to 4.5 hours after stroke onset,1 international guideline recommendations for tPA administration are within 3 hours.40-42 The risk of hemorrhagic transformation and worse clinical outcomes increase when tPA is administered beyond this therapeutic interval. Previous studies have demonstrated that exogenous tPA administration at 1 to 3 hours after MCAO in mice exerts neurotoxic and hemorrhagic effects.27,28,43,44 Our data suggest that inhibition of the contact system may extend the therapeutic time for tPA administration by reducing its hemorrhagic and neurotoxic effects.
Loss of BBB integrity soon after the onset of stroke is believed to be a precursor to brain edema and hemorrhagic transformation.45 MMP-9 has been implicated in tPA-induced cerebral hemorrhage and BBB disruption in ischemic stroke.6,46,47 We found that tPA infusion increases Evans blue dye extravasation in the ischemic hemisphere and PPK deficiency reduced this leakage. In addition, we also showed that PKal inhibition reversed the MMP-9 activity caused by tPA treatment. It is well established that plasmin activates MMP-2 and MMP-9, which consequently degrades laminin and collagen IV.48 Degradation of extracellular matrix could disrupt microvascular function and thereby increase BBB permeability.49,50 We have previously shown that PKal induces proteolysis of basement membrane and extracellular matrix proteins including multiple collagen isoforms, laminin β1 and γ1, nidogen 1 and 2, and fibronectin in brain astrocyte and pericytes.26,51 Moreover, PKal can interfere with collagen-induced platelet activation leading to intracerebral hematoma expansion in rodent models.11 These reports have revealed that PKal has collagen-binding and collagenase-like activities that might facilitate hemorrhage under certain pathological conditions. In intracerebral hemorrhage, increased plasma MMP-9 is associated with intracerebral hemorrhage enlargement52 and perihematoma edema.53 Higher baseline levels of blood MMP-9 are associated with increased risk of parenchymal hematoma after treatment with intravenous tPA.54,55 Taken together, these findings suggest that PKal inhibition during tPA therapy in stroke may reduce the upregulation of the MMP-9 pathway, which has been implicated in contributing to increased cerebral vascular permeability, intracerebral hemorrhage, and worse stroke outcomes.56 However, although MMP-9 contributes acutely to stroke injury it is also involved in brain plasticity and remodeling during recovery.57 Thus, reducing the overexpression of MMP-9 rather than blocking its basal activity may provide protective effects on the BBB.
Interestingly, we have also found that tPA exerted a greater effect on improving rCBF reperfusion following thrombotic MCAO in Klkb1−/− mice compared with WT mice. In addition, we show that coincubation of a PKal inhibitor with tPA increased the dissolution of clots induced by thrombin in vitro. Combined, these findings suggest that PKal inhibition may reduce thrombus stability and/or increase its susceptibility to tPA-mediated thrombolysis. These findings are consistent with recent evidence that PPK deficiency and PKal inhibition reduces arterial thrombosis induced by FeCl3 in animal models.58,59 Although the KKS appears to be dispensable for hemostatic mechanisms, inhibition of PKal may provide an opportunity to reduce cerebrovascular thrombotic disease without increasing tPA therapy-associated bleeding complications.60
A major limitation of this study is the use of mice with photothrombotic-induced MCAO as a model for clinical ischemic stroke. In humans, thrombotic stroke is usually the result of a thromboembolism, which often occurs in combination with underlying vascular disease and systemic disorders, such as diabetes and hypertension. It is unknown how composition of the thrombus and the mechanisms of intracerebral hemorrhage in this murine model compare with clinical stroke. In addition, humans and mice could differ in the relative contribution of coagulation factors involved in tPA-mediated complications and thrombus dissolution. We show that administration of tPA in mice at 2 hours after stroke increases brain injury and intracerebral hemorrhage, which is consistent with previous reports.27,28,43 However, this time is shorter than the 3 hours therapeutic window in humans. Species differences, faster metabolism, vascular damage caused by photothrombotic methodology, and tPA dose may contribute to the more rapid onset of tPA complications in mice.
In conclusion, the clinical benefits of tPA-mediated thrombolysis for ischemic stroke are still heavily dependent on time to treatment and limited by the risk of intracranial hemorrhage. It is imperative that pharmacological options are identified to reduce the risk of tPA-associated hemorrhagic transformation and expand the therapeutic window to enhance thrombolytic efficacy.29 In this report, we provide data suggesting that coadministration of a PKal inhibitor with tPA may provide an approach to improve safety and efficacy of tPA-mediated thrombolytic therapy for ischemic stroke.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Francis J. Castellino (University of Notre Dame, Notre Dame, IN) for providing the FXII-deficient mice.
This work was supported by grants from the National Institutes of Health, National Institute of Neurological Disorders and Stroke (NS077006) (E.P.F.) and the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (DK036836) (Joslin’s Diabetes Research Center grant).
Authorship
Contribution: F.S. designed the study, performed experiments, analyzed the data, and wrote the manuscript; T.U. and A.C.C. performed experiments and analyzed the data; and E.P.F. designed the study and wrote the manuscript.
Conflict-of-interest disclosure: E.P.F. is an employee of KalVista Pharmaceuticals Inc. (Cambridge, MA). The remaining authors declare no competing financial interests.
Correspondence: Edward P. Feener, Research Division, Joslin Diabetes Center, One Joslin Place, Boston, MA 02215; e-mail: edward.feener@joslin.harvard.edu.