Key Points
FL-infiltrating stromal cells overexpress CXCL12, which triggers FL B-cell migration, adhesion, and activation.
Polarization into CXCL12hi stroma involves IL-4+ TFH cells, unlike malignant B cells, revealing an indirect protumoral activity of FL-TFH cells.
Abstract
Follicular lymphoma (FL) is the most frequent indolent lymphoma and is characterized by the accumulation of germinal center–derived malignant B cells engaged in a bidirectional crosstalk with their supportive microenvironment in invaded lymph nodes (LNs) and bone marrow (BM). T follicular helper (TFH) cells and infiltrating stromal cells have been shown to favor FL B-cell growth, but the mechanisms of their protumoral effect and how the LN/BM microenvironment is converted into a lymphoma-permissive cell niche remain poorly understood. We demonstrated here that FL-infiltrating LN and BM stromal cells overexpressed CXCL12 in situ. Interleukin-4 high (IL-4hi) FL-TFH cells, unlike FL B cells themselves, triggered CXCL12 upregulation in human stromal cell precursors. In agreement, expression of CXCL12 was associated with IL-4 expression and signaling within the FL BM and LN niches. This IL-4/CXCL12 axis was amplified in activated lymphoid stromal cells as shown in our in vitro model of human lymphoid stroma differentiation and in an inducible mouse model of ectopic lymphoid organ formation. Finally, CXCL12 triggered primary FL B-cell activation, migration, and adhesion, a process antagonized by BTK and PI3K inhibitors. These data identified the IL-4/CXCL12 loop as a previously unrecognized pathway involved in lymphoid stroma polarization and as a potential therapeutic target in FL patients.
Introduction
Tumors are now considered a complex ecosystem in which the dynamic and mutualistic interactions between cancer cells and their surrounding microenvironment drive the coevolution of malignant cells and their supportive niche throughout the life history of the tumor.1 Cancer-associated fibroblasts (CAFs) have recently been recognized as key players in the cancer microenvironment and have critical roles in tumor initiation, progression, metastatic dissemination, immune escape, and drug resistance. CAFs make up a highly heterogeneous population of activated reprogrammed myofibroblasts that exhibit specific phenotype, proliferation rate, gene expression profile, and epigenetic features.2 They are supposedly derived from mesenchymal precursors, including mesenchymal stromal cells (MSCs), that could differentiate locally or are recruited from bone marrow (BM) or neighboring adipose tissue.3,4 Whereas tumor cells contribute to the polarization of protumoral CAFs, infiltrating immune cells also shape the recruitment and activation of stromal cells inside tumors.5 Myeloid cells were proposed as major drivers of stroma activation, but B and T cells could also modulate stroma functions. Such crosstalk has been well described in normal secondary lymphoid organs in which adaptive immune cells are known to produce factors, in particular tumor necrosis factor α (TNF-α) and lymphotoxin α1β2 (LT), which cooperate to support differentiation and maintenance of lymphoid stromal cells, including T-cell zone fibroblastic reticular cells (FRCs) and B-cell zone follicular dendritic cells (FDCs).6-8 CAFs can display some features of lymphoid stromal cells,9,10 but the precise relationship between CAFs and lymphoid stroma is not well understood.
Follicular lymphoma (FL), the most frequently occurring indolent lymphoma, is characterized by a long preclinical stage, a slow clinical course with multiple relapses, and a strong dependence on a specific microenvironment, including CD4+ T cells and stromal cells in particular.11,12 FL-infiltrating T cells are enriched for activated T follicular helper (TFH) cells, which are able to sustain survival of malignant B cells in vitro and are characterized by a specific cytokine profile.13,14 TFH-derived interleukin-4 (IL-4) has been shown to directly trigger FL B-cell activation as indicated by STAT6 phosphorylation,15 upregulation of surface immunoglobulin M,16 and induction of CCL22 and CCL17 secretion.17 Moreover, IL-4 could indirectly contribute to the polarization and organization of the FL protumoral cell niche. In particular, IL-4 is a well-known inducer of dendritic cell–specific intercellular adhesion molecule-3-grabbing nonintegrin, a mannose-binding lectin recently implicated in the antigen-independent triggering of FL BCR by tumor-associated macrophages.16 Whether IL-4 could modulate the properties of FL-infiltrating stromal cells has never been explored.
As expected for a malignancy resulting from the transformation of germinal center (GC) –derived B cells, FL-CAFs essentially display some features of lymphoid stromal cells.18 Accordingly, the typical BM infiltration pattern of FL is characterized by a local enrichment of CD4+ T cells19 and ectopic development of a heterogeneous population of lymphoid-like stromal cells.20,21 This emphasizes the pivotal role of these 2 cell subsets in FL pathogenesis and a potential common stromal program in FL lymph nodes (LNs) and BM. We previously demonstrated that MSCs obtained from FL BM (FL-MSCs) exhibit a specific gene expression profile compared with MSCs from healthy donor BM (HD-MSCs), including an enrichment of a lymphoid stroma signature associated with an increased capacity to sustain malignant B-cell growth.22 In addition, FL-MSCs produce higher levels of both CCL2 and IL-8, whose secretion is upregulated in HD-MSCs by FL B cells.22,23 These data indicate that a dynamic reciprocal cooperation program is activated between FL B cells and FL-infiltrating stromal cells, which results in the formation of the lymphoma-permissive stromal cell niche. Little is known about how lymphoma B cells get access to and are retained within their tumor niches inside LNs and BM, but both CXCR4 and CCR7 have been involved in these processes.24,25
Aiming at a better understanding of the FL-stroma protumoral phenotype, we unraveled a specific upregulation of CXCL12 in both LN- and BM-infiltrating stromal cells. Moreover, we revealed that CXCL12 induction was not related to the crosstalk with TNF-producing malignant B cells but with IL-4–producing FL-TFH cells. Interestingly, this IL-4/CXCL12 loop was also efficient in activated lymphoid-like stromal cells both in vitro and in vivo and should be considered a previously unrecognized pathway involved in lymphoid stroma polarization. Finally, the demonstration that CXCL12 favored malignant B-cell migration, adhesion, and activation argued for considering the IL-4/CXCL12 loop as a therapeutic target to disrupt FL protective cell niches.
Patients and methods
Human tissue and cell samples
Patients were recruited under institutional review board approval following the informed consent process according to the declaration of Helsinki and the French National Cancer Institute ethics committee recommendations. Lipoaspiration was performed in adults undergoing abdominal dermolipectomy. Adipose-derived stromal cells (ADSCs) were obtained as previously described from the stromal vascular fraction of adipose tissue and were maintained in a modified Minimum Essential Medium Eagle supplemented with 10% fetal calf serum (HyClone); 1 ng/mL of basic fibroblast growth factor (Cellgenix), penicillin, and streptomycin was used between passages 1 and 3.26 BM aspirates were obtained from FL patients and age-matched HDs undergoing cardiac surgery, BM plasma was frozen, and HD-MSCs and FL-MSCs were obtained as previously described.22 Tonsils were obtained from children undergoing routine tonsillectomy. LN and BM biopsies were also obtained from FL patients. Tonsil and FL B cells were purified by using B Cell Isolation Kit II (Miltenyi Biotec). FL-TFH cells were sorted by using a FACSAria cell sorter (BD Biosciences) as described.13
Immunohistofluorescence and immunohistochemistry
Tonsils, FL-LNs, and noninvaded/invaded FL-BM were embedded in O.C.T. compound (Tissue-Tek). Tonsils and FL-LN sections were analyzed for CXCL12, podoplanin, and CD21 long isoform (CD21L), and BM sections were analyzed for CXCL12 and CD20 expression. Immunohistochemistry for pSTAT6 and CXCL12 was applied to a tissue microarray of 195 formalin-fixed paraffin-embedded tissue specimens from 186 patients diagnosed with FL.27,28 For details, see the supplemental Data (available on the Blood Web site).
Chemokine quantification
CXCL12 was quantified on culture supernatants of ADSCs treated or not with TNF/LT or IL-4 and BM plasma obtained from HDs and FL patients by enzyme-linked immunosorbent assay (R&D Systems).
Real-time quantitative polymerase chain reaction
RNA was extracted from human cell suspensions and mouse tissues by using RNeasy Kit (Qiagen). Human tissue sections were homogenized by using QIAzol lysis reagent RNA (Qiagen), and RNA was extracted with phenol and chloroform. Complementary DNA was synthesized by using Superscript II reverse transcriptase, followed by real-time quantitative polymerase chain reaction (RT-qPCR). Assay-on-demand primers, probes, and TaqMan Universal Master Mix were obtained from Life Technologies. Gene expression was measured by using StepOnePlus (Life Technologies) based on a change in threshold cycle (ΔCt) calculation method. CDKN1B, EIF2B1, and PUM1 were determined to be appropriate internal controls for ADSCs, and B2M and CASC3 were determined to be appropriate internal controls for human LN and BM tissue samples by using TaqMan Endogenous Control Assays (Life Technologies). For mouse tissue samples, Pdgfrß was used as an endogenous control. For each sample, the Ct value for the gene of interest was determined and normalized to its respective mean value of housekeeping genes.
Stromal cell stimulation
ADSCs were treated by using TNF (20 ng/mL)/LT (100 ng/mL) or IL-4 (5 ng/mL) (R&D Systems) for 3 days. When indicated, ADSCs were cultured in the presence of TNF/LT for 3 days, and then cells were treated or not with IL-4 for 3 additional days. Purified FL-TFH cells (105 per mL) or FL B cells (5 × 105 cells per mL) were cocultured with confluent ADSCs for 48 hours before sorting of CD45–CD105+ DAPI– viable stromal cells and CXCL12 quantification by RT-qPCR.
Immunofluorescence
ADSCs stimulated with TNF/LT or IL-4 for 3 days were analyzed as described previously.29 In brief, cells were fixed in 2% paraformaldehyde and stained with rat anti-podoplanin and mouse anti-transglutaminase antibodies (Abcam) followed by labeling with anti-rat Alexa 488 and anti-mouse Alexa 594 secondary antibodies (Jackson ImmunoResearch). Coverslips were mounted by using Mowiol with Sytox blue (Life Technologies) and were examined with a confocal microscope (SP5X, Leica Microsystems). Digital images were analyzed by using ImageJ software.
Flow cytometry
Viable cells were analyzed after DAPI+ or TO-PRO-3+ cell exclusion (Life Technologies) by using the following monoclonal antibodies: fluorescein isothiocyanate–conjugated anti-CD19 and anti-CD20, phycoerythrin-conjugated anti-CD106/VCAM-1, and anti-CD54/ICAM-1 (Beckman Coulter), phycoerythrin-cyanin7–conjugated anti-CXCR4, and phycoerythrin-cyanin 5.5–conjugated anti-podoplanin/gp38 (eBioscience). Appropriate isotype-matched monoclonal antibodies were used to obtain mean fluorescence intensity ratios, and analyses were performed by using CyAn or Gallios (Beckman Coulter) flow cytometers.
Mice and salivary gland cannulation
Balb/c, Il4-rα−/−, and Il4−/− mice were bred and maintained under specific pathogen-free conditions in the Biomedical Service Unit at the University of Birmingham according to Home Office and local ethics committee regulations. The submandibular glands of female wild-type and knock-out mice (8-12 weeks old) were intraductally cannulated with 108 to109 plaque forming units (p.f.u.) of luciferase-encoding replication-defective adenovirus (Ad5), as previously described.30 Salivary glands were harvested at various time points after cannulation and used for evaluation of luciferase activity and quantification of gene expression.
Cell signaling
ADSCs were treated or not with TNF/LT for 24 hours, then starved for 2 hours in RPMI-1% fetal calf serum before stimulation with IL-4 (5 ng/mL) for 5 minutes. FL B cells were starved for 4 hours, treated or not for 30 minutes with ibrutinib (10 μM, Selleckchem), and stimulated by beads alone or beads coated with CXCL12-Fc at 30 μg/mL for 60 minutes. Analysis of IL-4–driven and CXCL12-driven signaling was performed as detailed in the supplemental Data.
Migration and adhesion assays
Purified FL B cells were treated or not with idelalisib/CAL101 (5 μM; Cayman Chemicals), ibrutinib (10 μM), or AMD3100 (1 μM; Sigma) for 30 minutes and were used for migration and adhesion to CXCL12 as detailed in the supplemental Data.
Statistical analysis
Statistical analyses were performed with Prism software version 6 (GraphPad Software) using the nonparametric Wilcoxon test for matched pairs or the Mann-Whitney nonparametric U test as appropriate. For association analysis between CXCL12 and pSTAT6 expression in human FL tissue biopsies, we performed a χ2 test.
Results
CXCL12 is upregulated within FL stromal cell niches
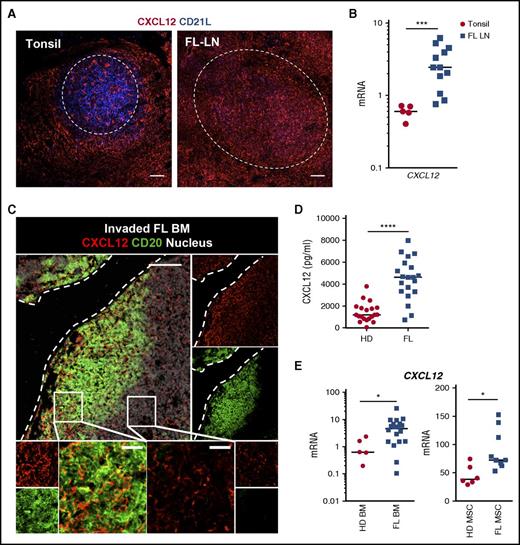
To decipher the driving signaling involved in the recruitment and homing of malignant FL B cells to their supportive cell niche, and given the key role of CXCL12 in normal GC organization, we first decided to evaluate the expression of CXCL12 by tumor-infiltrating stromal cells. In chronically inflamed tonsils, CXCL12-expressing stromal cells formed a dense meshwork in perifollicular and interfollicular areas, consisting of podoplanin/gp38+ FRCs (Figure 1A; supplemental Figure 2). Inside tonsil GCs, CXCL12 staining was always scarce and strictly restricted to CD21L– cells, whereas the CD21L+ mature FDC network was CXCL12-negative, as previously reported in mice.31 Conversely, immunohistofluorescence analysis revealed that CXCL12 was overexpressed in stromal cells within follicles in 8 of 14 FL patients and CXCL12 messenger RNA (mRNA) was significantly upregulated in FL LNs compared with tonsil samples (Figure 1A-B; supplemental Figure 3). Interestingly, CXCL12 overexpression was associated with a variable loss of the CD21L FDC marker as revealed by RT-qPCR and immunohistofluorescence experiments (supplemental Figure 3B).
Expression of CXCL12 within FL cell niches. (A) Tonsil and FL LN sections were stained with mouse immunoglobulin G1 (IgG1) anti-CXCL12 and mouse IgM anti-CD21L antibodies followed by appropriate secondary antibodies. Dotted lines indicate normal and malignant follicles. Scale bar, 100 µm. (B) CXCL12 was quantified by RT-qPCR in frozen tonsil (n = 5) and FL-LN (n = 12) sections. (C) Sections from invaded FL BM were stained with mouse IgG1 anti-CXCL12 and rabbit anti-CD20 antibodies followed by appropriate secondary antibodies. Nuclei were counterstained with SytoxBlue (white). Dotted lines indicate the bone. Scale bar, 100 µm. Boxes indicate the areas magnified in lower panels, including intratumoral zone (left) and extratumoral zone (right); scale bar, 20 µm. (D) CXCL12 concentration was measured by enzyme-linked immunosorbent assay (ELISA) in BM plasma from HDs (n = 20) and FL patients (n = 20). (E) CXCL12 was quantified by RT-qPCR in whole BM cells (left) and BM-MSCs (right) obtained from HDs (n = 5 and n = 6, respectively) and FL patients (n = 20 and n = 9, respectively). ****P < .0001; ***P < .001; *P < .05.
Expression of CXCL12 within FL cell niches. (A) Tonsil and FL LN sections were stained with mouse immunoglobulin G1 (IgG1) anti-CXCL12 and mouse IgM anti-CD21L antibodies followed by appropriate secondary antibodies. Dotted lines indicate normal and malignant follicles. Scale bar, 100 µm. (B) CXCL12 was quantified by RT-qPCR in frozen tonsil (n = 5) and FL-LN (n = 12) sections. (C) Sections from invaded FL BM were stained with mouse IgG1 anti-CXCL12 and rabbit anti-CD20 antibodies followed by appropriate secondary antibodies. Nuclei were counterstained with SytoxBlue (white). Dotted lines indicate the bone. Scale bar, 100 µm. Boxes indicate the areas magnified in lower panels, including intratumoral zone (left) and extratumoral zone (right); scale bar, 20 µm. (D) CXCL12 concentration was measured by enzyme-linked immunosorbent assay (ELISA) in BM plasma from HDs (n = 20) and FL patients (n = 20). (E) CXCL12 was quantified by RT-qPCR in whole BM cells (left) and BM-MSCs (right) obtained from HDs (n = 5 and n = 6, respectively) and FL patients (n = 20 and n = 9, respectively). ****P < .0001; ***P < .001; *P < .05.
To focus on the BM FL cell niche, we next performed immunohistofluorescence analysis on undecalcified biopsies from FL patients with and without BM involvement. In the absence of infiltrating malignant B cells, CXCL12 staining revealed a diffuse and homogeneous stromal cell network (supplemental Figure 4). BM sections from patients with BM invasion showed aggregates of CD20+ FL B cells with preferential paratrabecular localization. These B-cell–infiltrated areas displayed increased CXCL12 labeling compared with areas outside lymphoma infiltrates (Figure 1C). In agreement, CXCL12 quantification in BM plasma showed a fourfold increased CXCL12 level in FL BM (median, 4628 pg/mL; range, 726-7968 pg/mL; n = 20) compared with age-matched HD BM (median, 1192 pg/mL; range, 56-3796 pg/mL; n = 20, P<.0001) (Figure 1D). Moreover, FL BM cells demonstrated a higher expression of CXCL12 compared with BM cells obtained from age-matched HDs (Figure 1E). Finally, we checked CXCL12 levels in FL-MSCs, which have been proposed as a good in vitro model for studying lymphoma-driven alterations of the stromal microenvironment.22,23 Interestingly, we revealed that FL-MSCs exhibited a significantly higher expression of CXCL12 than HD-MSCs.
Altogether, these data argued for an upregulation of CXCL12 within FL stromal cell niches and raised the question of the mechanisms underlying this upregulation, including the factors involved and the inducer cell type.
IL-4, unlike TNF/LT, upregulates CXCL12 in stromal cells
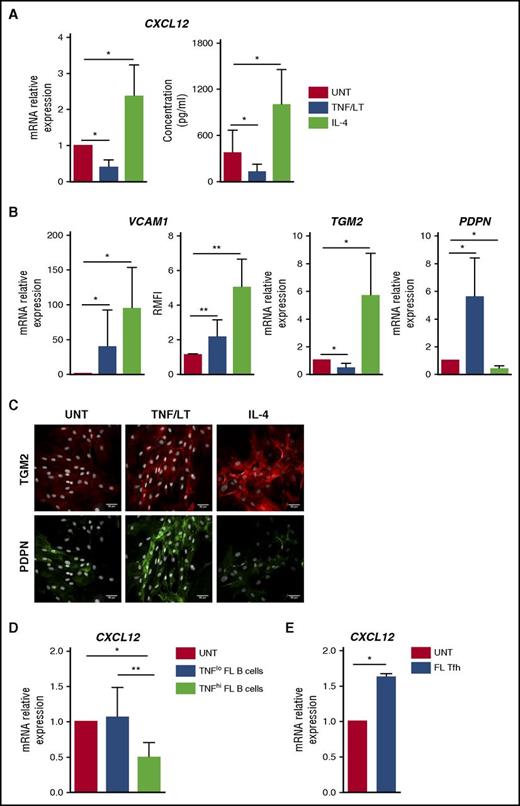
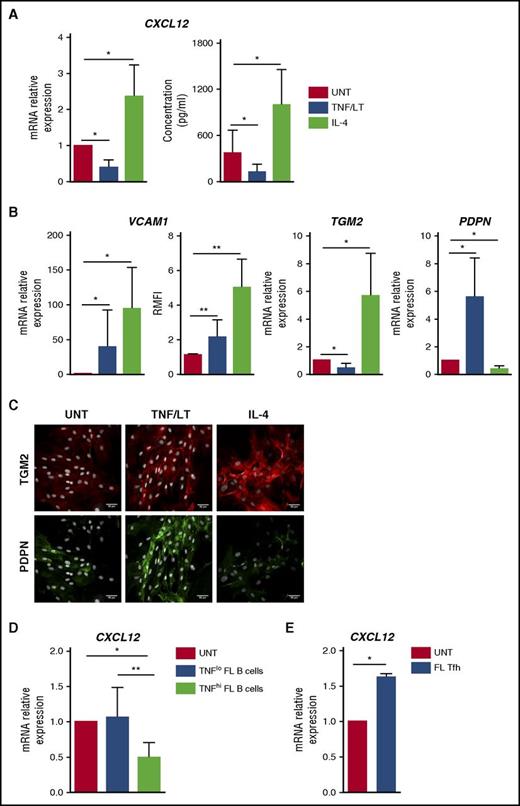
We previously demonstrated that human BM-MSCs could acquire in vitro a lymphoid-like stroma phenotype, associated with an upregulation of CCL19, BAFF, CCL2, and IL-8, in response to stimulation with TNF/LT.22,23,29 We thus decided to evaluate first how TNF/LT modulated CXCL12 production by MSCs. For that purpose, we developed a new in vitro model of lymphoid-stroma polarization based on ADSCs because adipose tissue has been identified as a precursor of lymphoid stroma for LNs and other lymphoid structures.32 Interestingly, human ADSCs, like BM-MSCs,29 were able to acquire an FRC-like phenotype upon stimulation with TNF/LT, including upregulation of ICAM-1/CD54, VCAM-1/CD106, gp38/podoplanin, BAFF, and CCL19, and construction of a dense extracellular meshwork of transglutaminase and podoplanin (supplemental Figure 5). In addition, ADSCs overexpressed CCL2 and IL8 in response to TNF/LT. In this validated model, we demonstrated a significant downregulation of CXCL12 at both mRNA and protein levels in ADSCs stimulated by TNF/LT (Figure 2A).
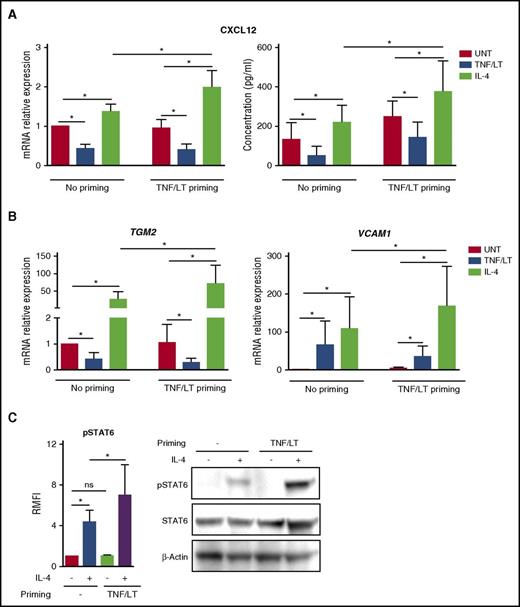
Characterization of stromal cell primed by TNF/LT vs IL-4. (A) CXCL12 was quantified at RNA (RT-qPCR; left) and protein (ELISA; right) levels in ADSCs treated by TNF/LT or IL-4 for 3 days. In RT-qPCR experiments, an arbitrary value of 1 was assigned to untreated cells (UNT). Results represent the mean ± standard deviation (SD) from 7 experiments. (B) ADSCs were stimulated by TNF/LT, IL-4, or left untreated (UNT) for 3 days before quantification of VCAM1, TGM2, and PDPN expression by RT-qPCR (n = 7). The arbitrary value of 1 was assigned to UNT cells. In addition, VCAM-1/CD106 expression was evaluated by flow cytometry (n = 5) and the ratio of mean fluorescence intensity (RMFI) was determined by comparison with an appropriate control isotype. (C) ADSCs were cultured for 3 days with TNF/LT, IL-4, or left untreated (UNT) before fixation and staining with transglutaminase and podoplanin. Nuclei were counterstained with SytoxBlue (white). Scale bars, 50 µm. (D) ADSCs were cocultured for 2 days with purified FL B cells expressing detectable (TNFhi; n = 6) or undetectable (TNFlo; n = 7) amounts of TNF as measured by ELISA, before sorting of CD45–CD105+DAPI– viable stromal cells and CXCL12 quantification by RT-qPCR. The arbitrary value of 1 was assigned to ADSCs cultured alone (UNT). (E) ADSCs were cocultured for 2 days with purified FL-TFH cells before sorting of CD45–CD105+ DAPI– viable stromal cells and CXCL12 quantification by RT-qPCR. Results represent the mean ± SD from 4 experiments. **P < .01; *P < .05.
Characterization of stromal cell primed by TNF/LT vs IL-4. (A) CXCL12 was quantified at RNA (RT-qPCR; left) and protein (ELISA; right) levels in ADSCs treated by TNF/LT or IL-4 for 3 days. In RT-qPCR experiments, an arbitrary value of 1 was assigned to untreated cells (UNT). Results represent the mean ± standard deviation (SD) from 7 experiments. (B) ADSCs were stimulated by TNF/LT, IL-4, or left untreated (UNT) for 3 days before quantification of VCAM1, TGM2, and PDPN expression by RT-qPCR (n = 7). The arbitrary value of 1 was assigned to UNT cells. In addition, VCAM-1/CD106 expression was evaluated by flow cytometry (n = 5) and the ratio of mean fluorescence intensity (RMFI) was determined by comparison with an appropriate control isotype. (C) ADSCs were cultured for 3 days with TNF/LT, IL-4, or left untreated (UNT) before fixation and staining with transglutaminase and podoplanin. Nuclei were counterstained with SytoxBlue (white). Scale bars, 50 µm. (D) ADSCs were cocultured for 2 days with purified FL B cells expressing detectable (TNFhi; n = 6) or undetectable (TNFlo; n = 7) amounts of TNF as measured by ELISA, before sorting of CD45–CD105+DAPI– viable stromal cells and CXCL12 quantification by RT-qPCR. The arbitrary value of 1 was assigned to ADSCs cultured alone (UNT). (E) ADSCs were cocultured for 2 days with purified FL-TFH cells before sorting of CD45–CD105+ DAPI– viable stromal cells and CXCL12 quantification by RT-qPCR. Results represent the mean ± SD from 4 experiments. **P < .01; *P < .05.
IL-4 is the most strongly deregulated cytokine in FL33 and is known to upregulate VCAM-1 on dermal fibroblasts.34 We thus tested the impact of IL-4 stimulation on ADSCs. Interestingly, IL-4 strongly increased the expression of VCAM-1 but also the production of CXCL12 in mesenchymal precursors (Figure 2A-B). In addition, whereas TNF/LT decreased transglutaminase mRNA, IL-4 triggered both transglutaminase expression and redistribution at the surface of stromal cells (Figure 2B-C). Finally, IL-4 reduced podoplanin expression by ADSCs. As a whole, whereas TNF/LT promoted ADSC in vitro differentiation into VCAM-1+podoplaninhiCXCL12lo cells, IL-4 favored a VCAM-1hipodoplaninloCXCL12hi phenotype, and both TNF/LT and IL-4 induced the construction of an extracellular transglutaminase-positive meshwork. FL-TFH cells were proposed as the major IL-4–producing cells within the FL cell niche,14 whereas malignant B cells produce no IL-4 but variably secrete TNF, previously involved in the induction of both CCL2 and IL-8 production by BM-MSCs.22,23 In agreement, coculture of stromal cells with TNFhi malignant B cells induced a decrease in CXCL12 expression. Conversely, purified CD4+CXCR5hiPD-1hi FL-TFH cells triggered an upregulation of CXCL12 in ADSCs (Figure 2D-E).
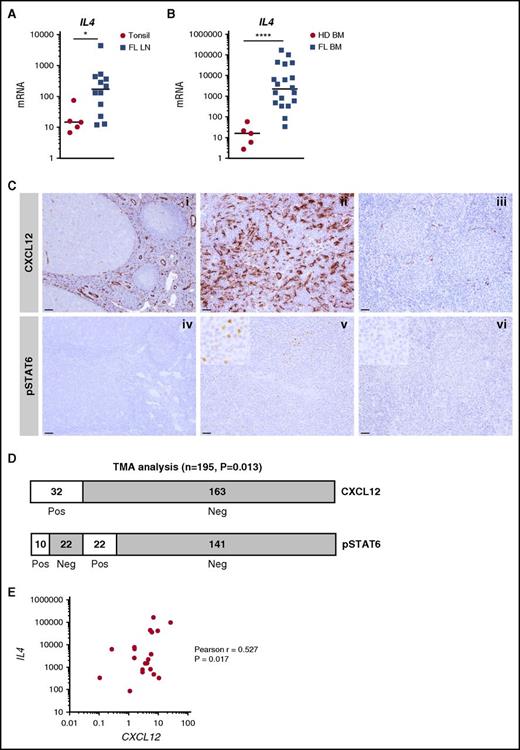
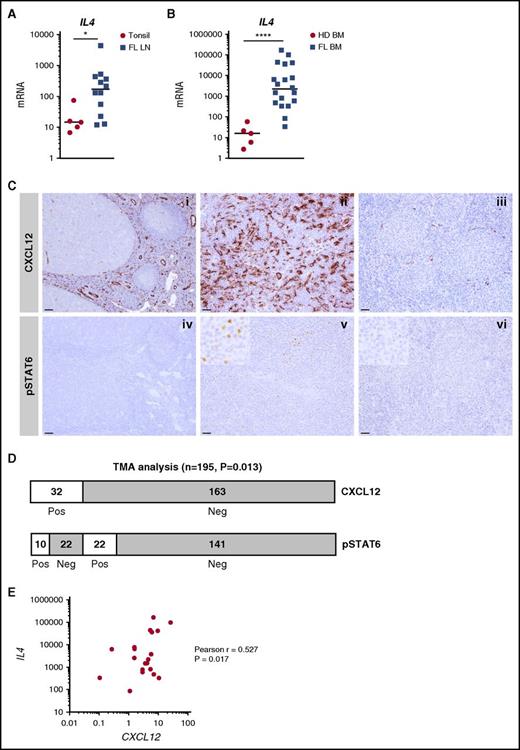
To further increase the relevance of these in vitro observations for FL biology, we evaluated the expression of IL-4 in situ in FL LNs and BM. IL4 mRNA was significantly upregulated in both FL cell niches (Figure 3A-B), unlike the IL-4–related cytokine IL13 (supplemental Figure 6). In agreement, by using a gene set enrichment analysis approach, we revealed that the enrichment for an IL-4 gene signature in sorted FL B cells compared with normal CD19+IgD–CD38hiCD10+CXCR4– tonsil centrocytes, thus confirming the activation of IL-4 signaling in FL (supplemental Figure 7). Because quantification of IL-4–producing cells in vivo is virtually impossible apart from using IL-4 reporter mice, we examined STAT6 phosphorylation together with CXCL12 staining across a large series of FL LN samples, as previously described27 (Figure 3C). We scored a sample as CXCL12 positive when CXCL12 expression within FL follicles was higher than that in reactive GCs used as on-slide controls. We then examined STAT6 phosphorylation in CXCL12-positive (n = 32) vs CXCL12-negative (n = 163) tumors. We observed a significant increase in STAT6 phosphorylation in the CXCL12-positive FL (P = .013; Figure 3D). Moreover, IL4 and CXCL12 expression were positively correlated across 20 FL BM samples (Figure 3E), thus further confirming the relationship between IL-4 and CXCL12 within FL cell niches. Interestingly, within FL LNs, transglutaminase was upregulated and podoplanin was downregulated (supplemental Figure 6), thus mimicking the phenotype of IL-4–treated stromal cells. Collectively, these results suggest that an IL-4/CXCL12 loop is activated within FL cell niches and that IL-4 contributes to the phenotype of FL stroma.
Expression and function of IL-4 within FL cell niches. (A) IL4 was quantified by RT-qPCR in frozen tonsil (n = 5) and FL-LN (n = 12) sections. (B) IL4 was quantified by RT-qPCR in whole BM cells from HDs (n = 5) and FL patients (n = 20). (C) (i-ii) Immunohistochemical staining for CXCL12 and pSTAT6 on reactive tonsil, (iii-iv) FL LN with high CXCL12/pSTAT6 expression, and (v-vi) FL LN with low CXCL12/pSTAT6 expression. Original magnification ×100 (i-ii: scale bars, 100 μm), ×200 (iii-vi: scale bars, 50 μm). (D) Semiquantitative assessment of CXCL12 and pSTAT6 on FL tissue biopsies arranged on tissue microarray (TMA). CXCL12 was scored positive when the expression was higher than in reactive GCs, and pSTAT6 was scored positive when frequent nuclear-positive cells within the malignant follicles were observed. (E) Correlation between IL4 and CXCL12 expression across the 20 FL BM samples. ****P < .0001; *P < .05.
Expression and function of IL-4 within FL cell niches. (A) IL4 was quantified by RT-qPCR in frozen tonsil (n = 5) and FL-LN (n = 12) sections. (B) IL4 was quantified by RT-qPCR in whole BM cells from HDs (n = 5) and FL patients (n = 20). (C) (i-ii) Immunohistochemical staining for CXCL12 and pSTAT6 on reactive tonsil, (iii-iv) FL LN with high CXCL12/pSTAT6 expression, and (v-vi) FL LN with low CXCL12/pSTAT6 expression. Original magnification ×100 (i-ii: scale bars, 100 μm), ×200 (iii-vi: scale bars, 50 μm). (D) Semiquantitative assessment of CXCL12 and pSTAT6 on FL tissue biopsies arranged on tissue microarray (TMA). CXCL12 was scored positive when the expression was higher than in reactive GCs, and pSTAT6 was scored positive when frequent nuclear-positive cells within the malignant follicles were observed. (E) Correlation between IL4 and CXCL12 expression across the 20 FL BM samples. ****P < .0001; *P < .05.
Lymphoid stroma commitment is associated with an increased IL-4–dependent CXCL12 production
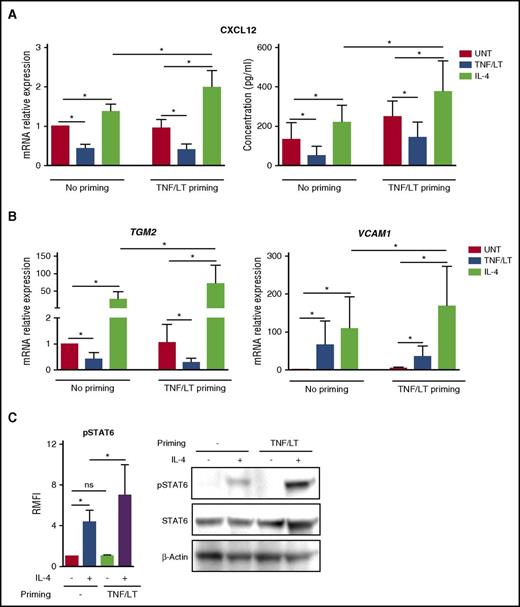
Having demonstrated that IL-4 could trigger CXCL12 expression by mesenchymal precursors, we decided to investigate its capacity to affect the production of CXCL12 by differentiated lymphoid stromal cells, thus mimicking the FL cell niche context. For that purpose, ADSCs previously committed to lymphoid stroma differentiation in vitro by priming with TNF/LT were treated with IL-4. As previously reported for resting stromal cells, FRC-like stromal cells upregulated CXCL12 in response to IL-4 at both mRNA and protein levels, whereas the TNF/LT combination retained its potential to decrease CXCL12 expression (Figure 4A). Strikingly, committed stromal cells could produce significantly higher levels of CXCL12 than their resting counterpart after stimulation by IL-4. Of note, the same results were obtained for transglutaminase and VCAM-1, both induced more efficiently on lymphoid-like cells than on ADSCs by exogenous IL-4 (Figure 4B). We next explored the mechanism of this increased sensitivity of lymphoid stroma to IL-4 signaling. IL-4 induced STAT6 phosphorylation in resting stromal cells but TNF/LT-pretreated stromal cells exhibited a significantly stronger STAT6 phosphorylation in response to IL-4, as shown by both flow cytometry and western blot (Figure 4C).
Impact of IL-4 on stromal cells committed to lymphoid stroma. (A-B) ADSCs were primed or not for 3 days with TNF/LT before treatment with TNF/LT, IL-4, or nothing (UNT) for 3 additional days and quantification of CXCL12, TGM2, and VCAM1 by RT-qPCR. The arbitrary value of 1 was assigned to ADSC maintained without any cytokine during all of the 6-day experiments. In addition, CXCL12 concentration was assessed by ELISA. Results represent the mean ± SD from 6 to 7 experiments. (C) ADSCs were primed or not for 24 hours with TNF/LT, starved for 2 hours, and then treated with IL-4 for 5 minutes. STAT6 activation was first evaluated by flow cytometry (left) and quantified as the RMFI of pSTAT6 staining in IL-4–treated vs IL-4–untreated cells (n = 6). In addition, STAT6, pSTAT6, and β-actin expression was determined by western blot, and 1 representative experiment of 2 is shown. ns, not significant. *P < .05.
Impact of IL-4 on stromal cells committed to lymphoid stroma. (A-B) ADSCs were primed or not for 3 days with TNF/LT before treatment with TNF/LT, IL-4, or nothing (UNT) for 3 additional days and quantification of CXCL12, TGM2, and VCAM1 by RT-qPCR. The arbitrary value of 1 was assigned to ADSC maintained without any cytokine during all of the 6-day experiments. In addition, CXCL12 concentration was assessed by ELISA. Results represent the mean ± SD from 6 to 7 experiments. (C) ADSCs were primed or not for 24 hours with TNF/LT, starved for 2 hours, and then treated with IL-4 for 5 minutes. STAT6 activation was first evaluated by flow cytometry (left) and quantified as the RMFI of pSTAT6 staining in IL-4–treated vs IL-4–untreated cells (n = 6). In addition, STAT6, pSTAT6, and β-actin expression was determined by western blot, and 1 representative experiment of 2 is shown. ns, not significant. *P < .05.
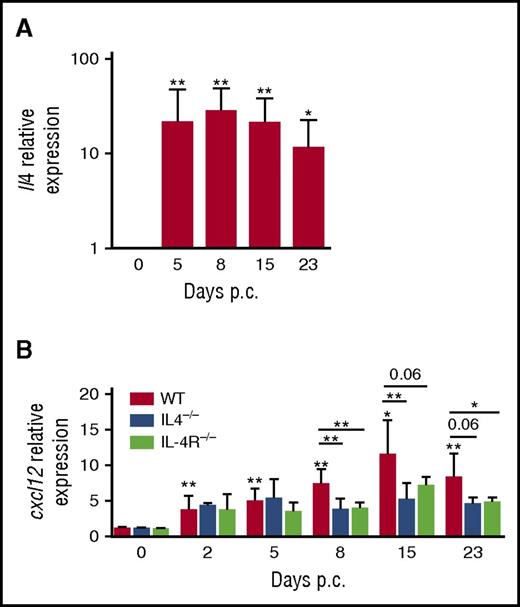
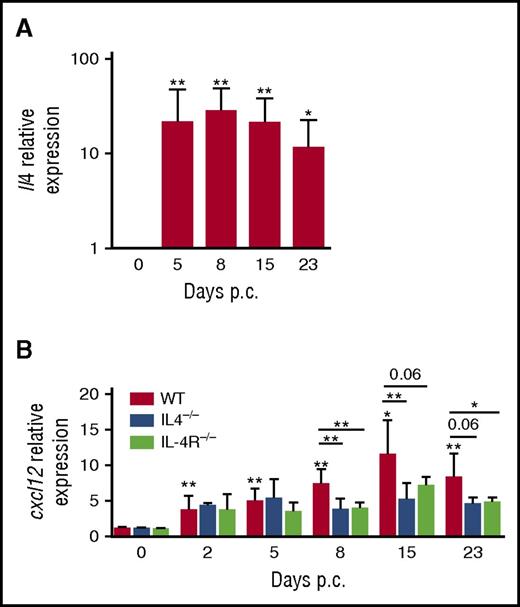
To investigate in vivo the relevance of this IL-4/CXCL12 loop in regulating lymphoid stroma function, we took advantage of an inducible mouse model of ectopic lymphoid organ formation that was induced by cannulation of murine salivary glands with a replication-deficient adenovirus and was associated with a significant increase of lymphoid chemokine expression.30 In wild-type mice, Il4 was strongly upregulated upon infection in cannulated glands with a peak at day 8 (Figure 5A). In parallel, Cxcl12 expression was significantly increased within salivary glands (Figure 5B). Interestingly, moving our analysis to Il4−/− and Il4r−/− mice revealed that a blockade of Il4 signaling pathway impaired CXCL12 expression from day 8 after cannulation. Taken together, these findings confirm that IL-4 has a role in the induction of CXCL12 in inflammatory nonmalignant lymphoid organs, phenocopying the observation in human FL samples.
IL-4/CXCL12 loop in a model of inducible ectopic lymphoid organs in mice. (A) RT-qPCR analysis of mRNA transcript for Il4 in wild-type (WT) mice on days 0, 5, 8, 15, and 23 days post cannulation (p.c.), normalized to Pdgfrß. Relative expression was calibrated to 0 day p.c. salivary glands. *P < .05; **P < .01 vs day 0. Results represent 2 experiments with 4 to 6 glands analyzed per group. (B) RT-qPCR analysis of mRNA transcript for Cxcl12 in WT mice, Il4−/− mice, and Il4r−/− mice on days 0, 5, 8, 15, and 23 p.c. normalized to Pdgfrß. Relative expression was calibrated to 0 days p.c. salivary glands. *P < .05; **P < .01 vs day 0. Results represent 2 experiments with 4 to 6 glands analyzed per group and are represented as the mean ± SD. Relative expression was calibrated to 0 day p.c. salivary glands. *P < .05; **P < .01.
IL-4/CXCL12 loop in a model of inducible ectopic lymphoid organs in mice. (A) RT-qPCR analysis of mRNA transcript for Il4 in wild-type (WT) mice on days 0, 5, 8, 15, and 23 days post cannulation (p.c.), normalized to Pdgfrß. Relative expression was calibrated to 0 day p.c. salivary glands. *P < .05; **P < .01 vs day 0. Results represent 2 experiments with 4 to 6 glands analyzed per group. (B) RT-qPCR analysis of mRNA transcript for Cxcl12 in WT mice, Il4−/− mice, and Il4r−/− mice on days 0, 5, 8, 15, and 23 p.c. normalized to Pdgfrß. Relative expression was calibrated to 0 days p.c. salivary glands. *P < .05; **P < .01 vs day 0. Results represent 2 experiments with 4 to 6 glands analyzed per group and are represented as the mean ± SD. Relative expression was calibrated to 0 day p.c. salivary glands. *P < .05; **P < .01.
CXCL12 mediates FL B-cell activation
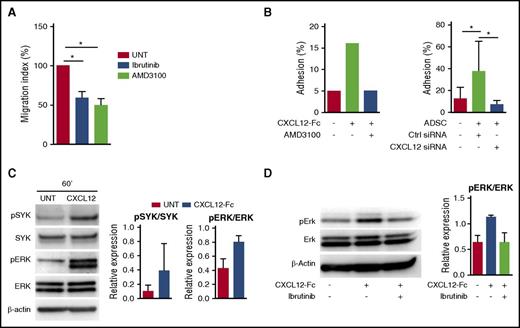
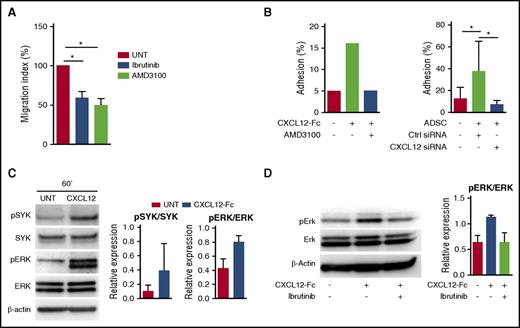
BCR activation is instrumental in the development and maintenance of most mature B-cell malignancies, leading to the recent development of therapeutic BCR inhibitors. Chemokine receptor signaling also involved various components of the BCR signaling pathways, and both BTK and phosphoinositide 3′-kinase isoform p110δ (PI3Kδ) inhibitors were shown to abrogate tumor cell migration toward stromal cell–derived CXCL12 in chronic lymphocytic leukemia (CLL).35,36 We recently pointed out the efficacy of the BTK inhibitor ibrutinib in abolishing the crosstalk between FL B cells and tumor-associated macrophages,16 but no study has been designed to decipher how ibrutinib or the PI3Kδ inhibitor idelalisib could affect stroma/FL B-cell interactions. We first evaluated CXCR4 expression in FL. Interestingly, purified FL B cells expressed significantly higher amounts of CXCR4 mRNA than their normal centrocyte counterparts (supplemental Figure 8A), but their membrane expression of CXCR4 was highly variable, as could be expected given the large amount of CXCL12 within the FL cell niche and the well-known ligand-dependent internalization of CXCR4 (data not shown). In agreement, as recently suggested,37 CXCR4 was re-expressed after 4-hour in vitro culture in the absence of CXCL12, a process that did not alter FL B-cell survival (supplemental Figure 8B). We then evaluated primary FL B-cell migration in response to CXCL12 after in vitro starvation and demonstrated that it was similarly reduced in response to the CXCR4 inhibitor AMD3100, ibrutinib, and idelalisib (Figure 6A). Interestingly, CXCL12 expression was also mandatory for triggering FL B-cell adhesion to stromal cells, as shown by blocking experiments based on the use of the CXCL12-Fc chimeric molecule or AMD3100 (Figure 6B). The same results were obtained by using CXCL12 small interfering RNA previously validated for their capacity to inhibit CXCL12 production by stromal cells (Figure 6B; supplemental Figure 9). CXCL12 was described as an activator of SYK, another key mediator of BCR signaling, and its downstream targets, including ERK, in CLL B cells.38 We demonstrated here that FL B cells could also respond to CXCR4 triggering by SYK and ERK phosphorylation (Figure 6C). In addition, ibrutinib was able to inhibit CXCL12-dependent ERK activation in primary FL B cells (Figure 6D). Of note, ibrutinib decreased slightly but significantly the expression of CXCR4 on primary FL B cells (supplemental Figure 8C).
CXCL12 signaling and functional impact on FL B cells. (A) Primary FL B cells pretreated or not (UNT) with the CXCR4 antagonist AMD3100, the BTK inhibitor ibrutinib, or the PI3Kδ inhibitor idelalisib were subjected to chemotaxis assay in response to CXCL12. Results are presented as the mean ± SD of the migration index (n = 6). (B) Primary FL B cells were labeled with carboxyfluorescein diacetate succinimidyl ester, treated or not with AMD3100, and incubated for 2 hours on either CXCL12-Fc–coated wells (left) or ADSC confluent cell monolayer (right). ADSCs were transduced 48 hours before by CXCL12 targeting small interfering RNA (siRNA) or control (Ctrl) siRNA. Adhesion percentage was calculated by comparing the residual fluorescence after adhesion with the fluorescence of the input. Shown is 1 representative experiment of 2 (left) or mean ± SD from 6 experiments (right). (C) Primary FL-B cells were starved for 4 hours and stimulated with uncoated (Ctrl) or CXCL12-Fc–coated beads for 60 minutes. Western blot revealed pSyk, Syk, pErk, Erk, and β-actin. Results are presented as the mean ± SD from 3 experiments, and 1 representative experiment is shown. (D) Primary FL B cells were starved for 4 hours and pretreated or not by ibrutinib before stimulation with uncoated or CXCL12-Fc–coated beads for 60 minutes and quantification of pErk, Erk, and β-actin by western blot. Results are presented as the mean ± SD from 4 experiments; 1 representative experiment is shown. *P < .05.
CXCL12 signaling and functional impact on FL B cells. (A) Primary FL B cells pretreated or not (UNT) with the CXCR4 antagonist AMD3100, the BTK inhibitor ibrutinib, or the PI3Kδ inhibitor idelalisib were subjected to chemotaxis assay in response to CXCL12. Results are presented as the mean ± SD of the migration index (n = 6). (B) Primary FL B cells were labeled with carboxyfluorescein diacetate succinimidyl ester, treated or not with AMD3100, and incubated for 2 hours on either CXCL12-Fc–coated wells (left) or ADSC confluent cell monolayer (right). ADSCs were transduced 48 hours before by CXCL12 targeting small interfering RNA (siRNA) or control (Ctrl) siRNA. Adhesion percentage was calculated by comparing the residual fluorescence after adhesion with the fluorescence of the input. Shown is 1 representative experiment of 2 (left) or mean ± SD from 6 experiments (right). (C) Primary FL-B cells were starved for 4 hours and stimulated with uncoated (Ctrl) or CXCL12-Fc–coated beads for 60 minutes. Western blot revealed pSyk, Syk, pErk, Erk, and β-actin. Results are presented as the mean ± SD from 3 experiments, and 1 representative experiment is shown. (D) Primary FL B cells were starved for 4 hours and pretreated or not by ibrutinib before stimulation with uncoated or CXCL12-Fc–coated beads for 60 minutes and quantification of pErk, Erk, and β-actin by western blot. Results are presented as the mean ± SD from 4 experiments; 1 representative experiment is shown. *P < .05.
Discussion
FL is a paradigm of microenvironment-dependent neoplasia, and infiltrating stromal cells have been proposed as key organizers of FL cell niches.18 However, specific features of FL CAFs in situ are relatively unexplored. A major emerging issue is thus to characterize FL stroma by looking for deregulation of tumor-supporting factors, to highlight the cellular and molecular determinants regulating this FL-specific stroma phenotype, and to evaluate the potential therapeutic impact of targeting the crosstalk between malignant B cells and their stromal cell niche.
Our study revealed that FL stromal cells variably but substantially upregulated CXCL12, the homeostatic chemokine supporting normal GC polarization into dark zone (DZ) and light zone (LZ).39 Of note, immunohistochemistry studies highlighted a lower percentage of FL samples harboring CXCL12hi follicular stromal cells than did immunohistofluorescence, but CXCL12 mRNA was found to be upregulated in the majority of FL patients, suggesting that CXCL12 deregulation is a recurrent feature in FL. Given the major role of normal GC B cells shuttling between DZ and LZ for their expansion, selection, and differentiation, CXCL12 is considered a critical regulator of GC responses, and its expression by lymphoid stromal cells is finely controlled. An intriguing question is thus to identify the origin of CXCL12hi stromal cells within FL LN follicles. Our study confirmed the disappearance of mature gp38hiCD21L+ FDCs in FL patients, an intriguing observation for a disease that targets centrocytes. Because FDCs are supposed to be sessile and long-lived cells, this loss of FDC markers in FL follicles was initially proposed to result from local FDC dedifferentiation.40 However, recent results revealed that the FDC network is more dynamic than previously anticipated, with a burst of FDC differentiation from highly proliferating progenitors in an inflammatory context.41 These data suggest that LZ FDCs could be replaced in FL patients by new stromal cells. Our first hypothesis was that CXCL12hi FL stromal cells arise from the so-called CXCL12-expressing reticular cells (CRCs) recently identified among gp38+CD21−/CD35− stromal cells of the GC DZ in mice.42 CRCs lack the main FRC and FDC markers and were thus proposed as a distinct cell type, even if they share a lineage precursor with both FRCs and FDCs.31 Apart from CRCs, mouse FRCs also express CXCL12, and CRC and FRC networks are tightly connected. Moreover, a subset of FRCs localized in follicular regions has recently been shown to contribute to B-cell homeostasis through the production of BAFF.43 Of note, we pinpointed that the gp38+CD21L– FRC network was dense and activated in FL patients. Whether CRCs or follicular FRCs could be considered as precursors of CXCL12hi FL stroma remains to be determined. Interestingly, FRC activation and expansion have been proposed to rely on the delivery of lymphotoxin beta receptor (LTβR)LTβR signals by incoming naïve B and T cells in response to inflammatory stimuli,44 and expression of LT by normal GC B cells is mandatory for mature FDC differentiation.45 Conversely, DZ CRCs do not require LT and TNF for maintenance of CXCL12 expression and network morphology.31 In agreement, purified FL B cells exhibit a downregulation of LTA and LTB compared with normal GC B cells (supplemental Figure 10), thus providing an argument in favor of a CRC-like origin for FL stromal cells. The origin of CXCL12hi FL stroma in invaded BM is even more puzzling. Within mouse BM, a subset of mesenchymal progenitors is characterized by a strong expression of CXCL12 and has been described as being in close contact with hematopoietic stem cells, B-cell progenitors, and long-lived plasma cells.46,47 We report here, for the first time, an upregulation of CXCL12 by human stromal cells admixed with ectopic malignant GC B cells. Unfortunately, the lack of relevant FL mouse model precludes cell tracking-based identification of FL stroma precursor cells.
Several mechanisms could cooperate in the modification of FL stromal cells, and we previously demonstrated that malignant B cells directly trigger upregulation of stromal CCL2 in a TNF-dependent manner.22 Interestingly, mouse B cells respond to the IL-4 burst associated with intestinal helminth infection by an increase in their expression of LT, which then interacts with LTβR+ FRCs to induce their expansion and reorganization.48 However, such a process is unlikely to take place in FL, given the downregulation of LT on malignant B cells. In agreement, we revealed that primary FL B cells were not able to stimulate in vitro CXCL12 production in stromal cells, whereas FL-TFH cells were efficient CXCL12 inducers. Several T-cell–derived cytokines could favor CXCL12 expression by lymphoid stromal cells. In particular IL-22 could upregulate CXCL13 and CXCL12 in tertiary lymphoid organs, thus favoring B-cell recruitment and autoantibody production.49 In addition, IL-17 drives the differentiation of IL-17Rc+ pulmonary stromal cells from bronchus-associated lymphoid tissues into gp38+CD21–/35–CXCL12+ follicular stromal cells allowing GC formation in the absence of both FDC and LT+ B cells.50 Altogether these data suggest that infiltrating T cells producing T helper 17 (Th17) –associated cytokines could contribute to the polarization of cancer-infiltrating stromal cells. Within the FL cell niche, malignant B cells skew the T regulatory cell/Th17 balance in favor of intratumoral T regulatory T cells,51 and FL-TFH cells poorly express Th17-related markers, including IL-17, IL-26, and RORC.13,14 IL-17 is thus probably not implicated in the differentiation of FL-specific stroma. Conversely, given the association between CXCL12 expression and IL-4 expression/signaling within FL cell niches, it was tempting to speculate that IL-4, the main FL-TFH–derived cytokine, could contribute to the stimulation of CXCL12 production by stromal cells. In agreement, we revealed for the first time that IL-4 could directly act on human mesenchymal progenitors and FRC-like cells to upregulate CXCL12 in the absence of LT-expressing B cells. Interestingly, IL-4 also triggered in vitro transglutaminase upregulation and gp38/podoplanin downregulation on stromal cells (a phenotype close to that identified in situ within the FL cell niche) and upregulated CD106, which was previously reported to rescue lymphoma B cells from rituximab-induced apoptosis.52 IL-4 thus exerts a wide effect on tumor-supportive FL stromal cells. The source of IL-4 within FL BM cells remains unknown, but even if their number is reduced compared with that in the LN niche, programmed cell death protein 1–positive T cells could be detected in FL BM together with an increase of the CD4/CD8 T-cell ratio, and these TFH/TFH-like cells are likely to be involved.19,53 In addition, IL-4 is also involved in the polarization of FL-infiltrating macrophages.16 Altogether, these results highlight IL-4 as a key mediator and FL pathogenesis and TFH-stroma crosstalk as a new B-cell extrinsic mechanism that supports FL cell growth (supplemental Figure 11).
IL-4 and TNF/LT similarly induced some FRC-like features in MSCs, but they exerted an antagonistic activity on CXCL12 and gp38/podoplanin expression, which raises questions regarding the underlying signaling pathways. Interestingly, CXCL13 is also regulated in an opposite way by TNF and IL-4 in monocytes and macrophages even if in this case TNF acts as an inducer and IL-4 as a repressor.54 In addition, TNF-induced classical nuclear factor κB signaling was already shown to inhibit CXCL12 expression in human umbilical vein endothelial cells55,56 and in a BM stromal cell line.52,53 The demonstration that TNF/LT-primed stromal cells became more sensitive to IL-4–dependent CXCL12 upregulation, at least in part through an increased expression of STAT6 signaling molecules, deserves further investigation.
In addition to their capacity to produce CXCL12, FL stromal cells have been proposed to contribute directly to FL B-cell growth through the expression of CD106, hedgehog family proteins, or BAFF, and indirectly through the recruitment of other components of the supportive FL cell niche, including tumor-associated macrophages and tumor-associated neutrophils.18,22,23 In agreement, targeting the crosstalk between malignant B cells and stromal cells is a promising therapeutic strategy. Among the possible approaches, the use of the BTK inhibitor ibrutinib or the PI3Kδ inhibitor idelalisib to impair CXCR4 signaling has been recently proposed in CLL,36,57 and here we demonstrated its relevance in FL. Other CXCR4 antagonists58 as well as SYK inhibitors38 are being evaluated for their capacity to counteract CXCL12 activity in B-cell malignancies and have yielded interesting in vitro results. Targeting CXCL12 specifically produced by a subset of pancreatic cancer CAFs is sufficient to override tumor immunosuppression in vivo and reveals the antitumor effects of anti-programmed death-ligand 1 immunotherapy.59 This finding may have important clinical relevance in FL in which immune-checkpoint blockade emerges as a valuable therapeutic approach. Finally, the use of JAK3 or STAT6 inhibitors (recently tested on CLL B cells60 ) may override the effect of IL-4 on the induction of CXCL12 expression by stromal cells and also on the activation of malignant B cells, especially in combination with inhibition of the BCR signaling pathway61 and would thus be of potential relevance in FL patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to the Centre de Ressources Biologiques-Santé (BB-0033-00056) of Rennes Hospital for its support in processing biological samples, Christophe Ruaux for providing tonsil samples, Erwan Flecher for providing normal bone marrow samples, and Nicolas Bertheuil for providing adipose tissues.
This work was supported by research grants from the Ligue Nationale Contre le Cancer (Equipe Labellisée and “Carte d’identité des Tumeurs” program) and the Institut National du Cancer (INCA AAP PLBIO-14-40). S.P. received a doctoral fellowship from the FP7 Marie Curie Initial Training Network (ITN 289720 Stroma) and the Ligue Nationale Contre le Cancer. A.M. was supported by fellowship awards from the Mildred-Scheel-Cancer Foundation, the Michael Smith Foundation for Health Research, and Lymphoma Canada. The immunofluorescence study was performed on the Microscopy Rennes Imaging Center (UMS 6480 Biosit, Rennes, France).
Authorship
Contribution: S.P. designed and performed experiments, analyzed data, and contributed to writing the manuscript; F.M. designed and analyzed data and contributed to writing the manuscript; T.M., S.N., M.G., C.P., C.M., P.A.-T., F.G., and J.D. performed experiments and analyzed data; T.F. provided samples; A.M. performed immunohistochemical studies; M.C. and F.B. designed and coordinated the mouse study; and K.T. designed and supervised research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karin Tarte, INSERM, UMR U1236, Faculté de Médecine, 2 Avenue du Pr Léon Bernard, F-35043 Rennes, France; e-mail: karin.tarte@univ-rennes1.fr.