Key Points
Isatuximab (anti-CD38 monoclonal antibody) given with lenalidomide/ dexamethasone is active in heavily pretreated relapsed/refractory myeloma
Overall, the safety profile of this combination is similar to the characteristic safety profiles of the individual agents.
This phase 1b, open-label, dose-escalation study assessed the safety, efficacy, and pharmacokinetics of anti-CD38 monoclonal antibody isatuximab given in 2 schedules (3, 5, or 10 mg/kg every other week [Q2W] or 10 or 20 mg/kg weekly [QW] for 4 weeks and then Q2W thereafter [QW/Q2W]), in combination with lenalidomide 25 mg (days 1-21) and dexamethasone 40 mg (QW), in patients with relapsed/refractory multiple myeloma (RRMM). Patients received 28-day treatment cycles; the primary objective was to determine the maximum tolerated dose (MTD) of isatuximab with lenalidomide and dexamethasone. Fifty-seven patients (median 5 [range 1-12] prior regimens; 83% refractory to previous lenalidomide therapy) were treated. Median duration of dosing was 36.4 weeks; 15 patients remained on treatment at data cutoff. Isatuximab-lenalidomide-dexamethasone was generally well tolerated with only 1 dose-limiting toxicity reported (grade 3 pneumonia at 20 mg/kg QW/Q2W); the MTD was not reached. The most common isatuximab-related adverse events were infusion-associated reactions (IARs) (56%), which were grade 1/2 in 84% of patients who had an IAR and predominantly occurred during the first infusion. In the efficacy-evaluable population, the overall response rate (ORR) was 56% (29/52) and was similar between the 10 mg/kg Q2W and 10 and 20 mg/kg QW/Q2W cohorts. The ORR was 52% in 42 evaluable lenalidomide-refractory patients. Overall median progression-free survival was 8.5 months. Isatuximab exposure increased in a greater than dose-proportional manner; isatuximab and lenalidomide pharmacokinetic parameters appeared independent. These data suggest that isatuximab combined with lenalidomide and dexamethasone is active and tolerated in heavily pretreated patients with RRMM. This trial was registered at www.clinicaltrials.gov as #NCT01749969.
Introduction
Over the past decade, significant improvements have been made in the treatment of patients with multiple myeloma (MM). New therapies, including proteasome inhibitors (PIs; bortezomib [BORT], carfilzomib [CAR], and ixazomib), immunomodulatory drugs (IMiDs; thalidomide, lenalidomide [LEN], and pomalidomide [POM]), and histone deacetylase inhibitors (panobinostat and vorinostat), have been used in various combinations as both first- and later-line treatments, resulting in improved response rates and longer progression-free survival (PFS) compared with traditional therapies.1,,,,,,-8 More recently, 2 first-in-class monoclonal antibodies (mAbs) with novel mechanisms of action (daratumumab [DARA]9,10 and elotuzumab [ELO]11,12 ) have been approved for use in the treatment of patients with relapsed/refractory multiple myeloma (RRMM) and have generated considerable enthusiasm for the use of immunotherapies for this malignancy.
ELO is a humanized, “naked,” immunoglobulin G1 (IgG1) mAb that targets SLAMF7, an antigen highly expressed on malignant plasma cells.13 Potent anti-MM activity was demonstrated in a phase 3 trial comparing ELO, LEN, and dexamethasone (Dex) against LEN and Dex in patients with RRMM who had received 1 to 3 prior lines of therapy.14 The other approved mAb, DARA, is a human IgG1 mAb that targets CD38, a multifunctional cell surface protein widely expressed on malignant plasma cells. DARA received initial approval following a large phase 2 single-arm monotherapy trial showing an overall response rate (ORR) of 29% in patients with heavily pretreated RRMM who had received at least 3 lines of therapy (including PIs and IMiDs) or were refractory to PIs and IMiDs.15 More recently, 2 large phase 3 trials (CASTOR and POLLUX) demonstrated prolonged PFS and improved response rates with DARA in combination with either BORT-Dex or LEN-Dex vs the standard doublets (BORT-Dex and LEN-Dex) in patients with RRMM who had received at least 1 prior therapy.16,17 DARA has now been approved in combination with either of these doublets for the treatment of RRMM.9 The studies of ELO and DARA have demonstrated the potent effects of mAb therapies against MM, supporting the development of additional agents in this class, and in particular, testing their potential activity in heavily pretreated and LEN-refractory patients.
Isatuximab (SAR650984) is a chimeric IgG1 mAb made by variable domain resurfacing.18 Isatuximab binds to a unique epitope on human CD38, targeting a completely different amino acid sequence than DARA.19 Preclinical studies indicated that isatuximab elicits anti-MM activity through multiple mechanisms, including direct induction of apoptosis, antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, and complement-dependent cytotoxicity.19 In addition, isatuximab inhibits the ecto-enzymatic activity of CD38, thereby altering calcium homeostasis and potentially eliciting anticancer effects.19 Initial data suggest that isatuximab has clinical activity as a single agent, with an ORR of 24% to 29% obtained in patients with RRMM in the highest dose cohorts of a phase 2 study20 (International Myeloma Working Group [IMWG] response criteria21 ; NCT01084252).
In xenograft MM models, isatuximab demonstrated synergistic anticancer activity with LEN with no increase in toxicity,22 suggesting that the immunostimulatory activity of LEN on T cells and natural killer cells23,-25 may enhance isatuximab-mediated tumor cell killing. We therefore conducted a phase 1b dose-escalation study (NCT01749969) to investigate the combination of isatuximab with LEN and Dex in patients with RRMM. The final results of this phase 1b study are reported here.
Methods
This study was conducted in 2 parts: originally, isatuximab was administered every other week (Q2W) in dose-escalation cohorts at 3, 5, or 10 mg/kg. The protocol was subsequently amended to evaluate isatuximab 10 or 20 mg/kg given weekly (QW) for 4 doses and then Q2W thereafter.
Study population
For the isatuximab Q2W cohorts, patients had a confirmed diagnosis of MM, had progressed or were refractory to the immediate prior therapy, and had received ≥2 prior anti-MM regimens. For the isatuximab QW/Q2W cohorts, eligibility criteria were amended so that only patients who had received ≥2 lines of anti-MM therapy (line defined as ≥1 planned cycle of single-agent or combination therapy, or a sequence of treatments in a planned manner26 ) and were LEN-exposed were included. RRMM was defined according to the International Myeloma Workshop guidelines26 ; a regimen was defined as a distinct single-agent or combination therapy used for >1 cycle of anti-MM treatment. Eligible patients were aged ≥18 years, with Karnofsky performance status ≥60%; absolute neutrophil count ≥1.0 × 109/L; platelet count ≥75 × 109/L (>30 × 109/L in patients with ≥50% bone marrow plasma cells); hemoglobin ≥10 g/dL; alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and bilirubin ≤1.5 × upper limit of normal; creatinine clearance ≥30 mL/min; and fasting glucose ≤150 mg/dL. Patients had measurable disease, with serum M-protein ≥0.5 g/dL, urine M-protein ≥200 mg per 24 hours, or involved serum free light chain assay ≥10 mg/dL and an abnormal serum free light chain ratio (<0.26 or >1.65), or biopsy-proven plasmacytoma. Key exclusion criteria included prior autologous transplantation within 12 weeks; prior anticancer treatment within 21 days; prior active malignancy; daily requirement for corticosteroids (>10 mg/d of prednisone); history of active bleeding or a severe underlying medical condition; prior major surgery within 4 weeks; active hepatitis B, C, or known HIV infection; baseline neuropathy grade ≥3 or painful neuropathy grade ≥2; pregnancy or breast-feeding; or hypersensitivity to any components of study therapy.
Study design
This study was a phase 1b, multicenter, open-label, dose-escalation study conducted at 5 US centers. Isatuximab was administered IV as 1 of 2 dosing schedules (in a staggered approach) in combination with standard doses of oral LEN 25 mg (10 mg if creatinine clearance ≤60 mL/min; days 1-21) and Dex 40 mg (days 1, 8, 15, and 22), in continuous 28-day cycles. For the Q2W schedule, isatuximab was administered on days 1 and 15 of each cycle in dose-escalation cohorts at 3, 5, or 10 mg/kg. For the QW/Q2W schedule, isatuximab was evaluated at 10 or 20 mg/kg QW during cycle 1 and then Q2W thereafter (QW/Q2W). The planned initial infusion rate was 175 mg/h in the Q2W cohorts, 250 mg/h in the 20 mg/kg QW/Q2W cohort, and either 175 or 250 mg/h in the 10 mg/kg QW/Q2W cohort. Dose cohorts were enrolled sequentially using a standard 3+3 design to establish the maximum tolerated dose (MTD; highest dose level at which <2 of 6 patients experienced a dose-limiting toxicity [DLT]). Once the MTD was reached, or the highest planned dose was achieved safely, expansion cohorts were to be enrolled for both schedules (18 patients planned for 1 expansion cohort for the Q2W schedule; for the QW/Q2W schedule, expansion of up to 12 patients was planned for both doses tested) for further safety and preliminary efficacy evaluation of this combination. Patients continued treatment until unacceptable toxicity, progressive disease, withdrawal of consent, or investigator discretion.
Nonhematological DLTs were defined as any grade ≥3 adverse event (AE) excluding grade 3 fatigue, grade 3/4 electrolyte abnormalities (including hyperglycemia), grade 3 nausea/vomiting/diarrhea responsive to medical management within 48 hours, or any allergic reaction/hypersensitivity reaction attributed to isatuximab. Grade 3 neuropathy was also excluded as a DLT if it occurred in enrolled patients who already had baseline grade 2 neuropathy without pain. Hematological DLTs were grade 4 neutropenia lasting ≥7 days, grade 3/4 neutropenia complicated by fever or infection, grade 3/4 thrombocytopenia associated with bleeding requiring platelet transfusion, or any treatment delay for >14 days due to hematological toxicity.
To mitigate infusion-associated reactions (IARs), the protocol mandated use of standard premedications (Dex 40 mg IV or methylprednisolone 100 mg IV; diphenhydramine 50 mg IV or equivalent; ranitidine 50 mg IV or equivalent; and acetaminophen 650-1000 mg oral administration) up to 60 minutes before each isatuximab infusion. Dex is part of the combination regimen, so was used as both premedication and backbone therapy. Prophylaxis against thromboembolic complications was recommended (acetylsalicylic acid 81-325 mg daily or other agents, eg, Coumadin or low-molecular-weight heparin).
Endpoints and assessments
The primary objective was to determine the MTD of isatuximab in combination with LEN and Dex in patients with RRMM. Secondary objectives included evaluation of safety, pharmacokinetics (PK), and efficacy.
The severity of AEs was assessed according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). Treatment-emergent AEs were defined as AEs that developed, worsened (investigator’s judgment), or became serious during the on-treatment phase. Disease responses were determined by investigator assessment according to the IMWG response criteria.21 All responses and progressions were to be confirmed at a subsequent assessment. For the patients in the Q2W dosing cohorts, high-risk MM was determined based on the presence of 1 or more of the following: deletion of the short arm of chromosome 17 [del(17p)] (by fluorescence in situ hybridization [FISH]), translocations between chromosomes 4 and 14 [t(4;14)] (FISH), chromosome 13 monosomy (cytogenetics), or hypodiploidy (cytogenetics), according to central review (Mayo Clinic, Phoenix, AZ) of local data. High-risk patients in the QW/Q2W cohorts were determined by the presence of del(17p) or t(4;14) (FISH) at a central laboratory.
Blood samples were taken at selected time points for PK evaluation of isatuximab (days 1, 2, 3, 4, 8, and 15) and LEN (days 1, 2, 15, and 16). Isatuximab concentrations were determined using a validated enzyme-linked immunoadsorption assay with a lower limit of quantification of 0.5 ng/mL, whereas a validated liquid chromatography/tandem mass spectrometry method was used to measure LEN concentrations (lower limit of quantification: 5 ng/mL). PK parameters after the first and repeated administration (LEN only) were determined by noncompartmental analysis using WinNonlin software version 5.2.1 (Pharsight).
Study oversight
The study protocol was approved by the ethics committee at every institution and was conducted in accordance with recommendations of Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent before participation in the study. This study was registered at Clinicaltrials.gov (NCT01749969).
Statistical considerations
All analyses were performed for the all-treated/safety population (patients who received at least 1 dose [even if incomplete] of isatuximab, LEN, or Dex), and efficacy assessments were also taken for the efficacy-evaluable population (patients who received at least 1 cycle of treatment). The PK population consisted of all patients in the safety population who had an evaluable PK parameter. Continuous data were summarized using mean, standard deviation (SD), median, and range. Categorical and ordinal data were summarized using number and percentage. Response assessments included ORR (at least partial response [PR]), according to the IMWG criteria.21 PFS was defined as the time from the first dose of study medication to disease progression or death, whichever came first. In the absence of disease progression or death before the analysis cutoff date, PFS was censored at the date of the last valid assessment before the cutoff date. PFS was analyzed using the Kaplan-Meier method.
Results
Patients and treatment
Between February 2013 and August 2015, a total of 57 patients were treated with isatuximab in combination with standard doses of LEN and Dex. Twenty-four patients were treated in the dose-escalation phase (3 mg/kg Q2W, n = 4; 5 mg/kg Q2W, n = 3; 10 mg/kg Q2W, n = 6; 10 mg/kg QW/Q2W, n = 3; 20 mg/kg QW/Q2W, n = 8). Three patients were replaced for DLT evaluation due to grade 3 IARs that occurred during the first isatuximab infusion and led to treatment discontinuation (1 patient at 3 mg/kg Q2W, and 2 patients at 20 mg/kg QW/Q2W). As permitted in the protocol, 6 patients were treated at 10 mg/kg to confirm safety and tolerability before opening the QW/Q2W expansion cohorts. A further 33 patients were treated in the dose-expansion phase (10 mg/kg Q2W, n = 18; 10 mg/kg QW/Q2W, n = 9; 20 mg/kg QW/Q2W, n = 6). The cutoff date for this analysis was 26 May 2016 (9 months after last patient in). Results presented here are final; patients still on treatment at the data cutoff date are to be followed up for safety assessment only. Baseline patient demographics, disposition, and disease characteristics are summarized in Table 1. The median age was 61 years (range 42-76 years), with 20 patients (35%) aged >65 years. The median time from MM diagnosis was 4.3 years. In patients with available cytogenetic/FISH data, 15 (26%) were classified as high risk (1 or more of del(17p),t(4;14), chromosome 13 monosomy, or hypodiploidy). Overall, patients had received a median of 7 (range 2-15) prior anti-MM regimens and a median of 5 (range 1-12) previous lines of therapy; 15 patients (26%) had received ≥8 prior lines of treatment. Fifty-three patients (93%) had received prior PI and IMiD therapy, and 39 (68%) had received POM or CAR. A total of 54/57 patients (95%) had received a stem cell transplant, and all except 1 (56/57 [98%]) had received an alkylating agent. Before study entry, 47 patients (82%) were refractory to LEN (42 patients refractory to their last regimen containing LEN; refractory status defined according to IMWG criteria26 ), and 50 (88%) were refractory to any IMiD-based therapy (LEN, POM, or thalidomide) (Table 1). Overall, 86% of patients were refractory to their most recent anti-MM treatment.
Patients received a median of 9 cycles (range 1-37) of isatuximab-LEN-Dex therapy, with 21 patients (37%) receiving at least 12 cycles. A total of 1174 isatuximab infusions had been started. The median time on study treatments was 36.4 weeks (range 1-152 weeks) and was longer for the isatuximab 10 mg QW/Q2W cohort (50.8 weeks) than the 10 mg/kg Q2W cohort (25.8 weeks) or 20 mg/kg QW/Q2W cohort (10.1 weeks). At the analysis cutoff date, 42 patients had discontinued study treatment, including 29 patients because of disease progression and 9 patients due to AEs. Fifteen patients remained on treatment with at least isatuximab at data cutoff. All patients were analyzed for safety and 52 patients were included in the efficacy-evaluable population (5 patients discontinued in cycle 1 before efficacy measurements could be completed: 4 in the 20 mg/kg QW/Q2W cohort and 1 in the 3 mg/kg Q2W cohort).
Safety
Only 1 DLT was observed in the dose-escalation part of the study (grade 3 bilateral pneumonia in the 20 mg/kg QW/Q2W cohort), which resolved following treatment discontinuation. The MTD was not reached. Overall, 50/57 patients (88%) had at least 1 grade 3/4 treatment-emergent AE, and treatment-emergent serious AEs were recorded in 32/57 patients (56%). Besides IARs, the most common treatment-emergent AEs (all grades) were diarrhea (53%), fatigue (49%), upper respiratory tract infection (40%), and nausea (35%), all of which were grade <3 in severity except 4 cases of fatigue (Table 2). Grade ≥3 treatment-emergent AEs observed in >1 patient were pneumonia (9%), fatigue (7%), febrile neutropenia, lung infection, anaphylactic reaction, hypokalemia (5% each), hypertension, hypotension, dyspnea, hyperglycemia, hyponatremia, bronchospasm, and facial edema (4% each) (Table 2). Pulmonary embolism (grade 3) and deep vein thrombosis (grade 1/2) were observed in 1 patient each in the 10 mg/kg Q2W cohort; no other thromboembolic events were reported. The most frequent grade 3/4 hematological laboratory abnormalities during treatment were neutropenia (60%), lymphopenia (58%), leukopenia (53%), and thrombocytopenia (38%) (Table 2).
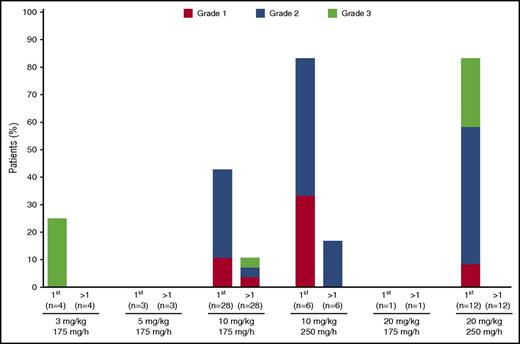
IARs were the most common isatuximab-related AEs, observed in 32 patients (56%). IARs occurred only with the first infusion in 28 of the 32 patients (88%) (Figure 1), and no IARs were recorded after the fourth infusion. IARs were predominantly grade 1/2 in severity; 5 patients had grade 3 IARs, and no patient had grade 4 IARs. The 5 patients with grade 3 IARs discontinued treatment due to anaphylactic reaction and bronchospasm (n = 1; 3 mg/kg Q2W); maculopapular rash (n = 1; 10 mg/kg Q2W); anaphylactic reaction (n = 2, 20 mg/kg QW/Q2W); and bronchospasm (n = 1; 20 mg/kg QW/Q2W). All IARs leading to discontinuation occurred with the first infusion except for the case of maculopapular rash, which occurred with the third infusion; all resolved after treatment discontinuation. The starting infusion rate was 250 mg/h in 3 of the patients who discontinued treatment due to IARs. In the cohorts examined in the dose-expansion part of the study, IARs were more common at the 250 mg/h initial isatuximab infusion rate (83% at this rate) compared with the 175 mg/h initial infusion rate (50%), prompting the use of the 175 mg/h initial infusion rate across the isatuximab program. Temporary infusion interruption due to an IAR was required in 22 patients (39%). The median infusion duration for the first infusion was 3.7 and 3.1 hours (range 2-7 hours) for the isatuximab 10 mg/kg Q2W and QW/Q2W cohorts, respectively, and 4.9 (range 0-11) hours for isatuximab 20 mg/kg. Median duration for subsequent infusions was slightly shorter for both doses: 2.3-2.4 (0-4) and 4.6 (1-26) hours at 10 and 20 mg/kg, respectively.
IARs by grade and infusion number. IARs during first infusion or after first infusion are displayed for each dose, according to initial infusion rate. No IARs were observed after the fourth infusion.
IARs by grade and infusion number. IARs during first infusion or after first infusion are displayed for each dose, according to initial infusion rate. No IARs were observed after the fourth infusion.
In addition to the patients who discontinued treatment due to IARs, 4 other patients discontinued study treatment due to other treatment-emergent AEs (bacterial sepsis; intraoperative hemorrhage; fatigue; pneumonia). There were 5 on-treatment deaths (≤30 days after last dose of study treatments): 2 due to AEs not related to study treatments (1 due to bacterial sepsis; the other due to an intraoperative hemorrhage) and 3 due to disease progression.
Efficacy
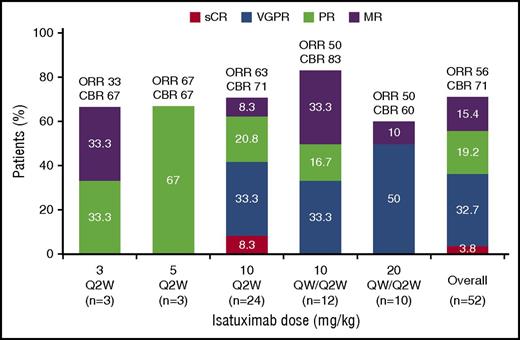
With an overall median follow-up of 9 months, the ORR for the all-treated population was 51%, including 2 patients with stringent complete response, 17 with very good partial response (VGPR), and 10 with PR (all responses were subsequently confirmed). A further 8 patients attained minimal response (MR26 ), so the clinical benefit rate (CBR; at least MR) for the all-treated population was 65%. In the efficacy-evaluable population (n = 52; excluding patients who discontinued before completing at least 1 cycle of treatment), the ORR was 56% and CBR was 71% (Figure 2). Overall, the ORR was generally similar across the expansion cohorts for the efficacy-evaluable population (63%, 50%, and 50% for the 10 mg/kg Q2W, 10 mg/kg QW/Q2W, and 20 mg/kg QW/Q2W cohorts, respectively). Of note, among 42 efficacy-evaluable patients who were refractory to LEN, the ORR was 52% and CBR was 67% (Figure 3). Overall, the ORR was 51% for efficacy-evaluable patients refractory to an IMiD (n = 45). Analysis of response according to the number of prior lines of therapy revealed that all efficacy-evaluable patients who had received 1 to 2 lines (n = 8) attained at least a VGPR. The ORR was 48% in patients who had received ≥3 previous treatment lines (n = 44) (Figure 3). In patients with high-risk cytogenetics in the efficacy-evaluable population (n = 13; 10 patients from the Q2W dosing cohorts with central review of local data, and 3 patients from the QW/Q2W cohorts with assessment at a central laboratory), the ORR was 31% and the CBR was 38%. For the efficacy-evaluable population, the median time to first response was 0.95 months (range 0.9-4.1 months) and was similar across all dose-expansion cohorts (0.95-0.99 months). The median duration of response (at least PR) was 10.9 months (range 1.4-31.4 months) for the efficacy-evaluable population and was 13.01, 10.28, and 8.54 months for the 10 mg/kg Q2W, 10 mg/kg QW/Q2W, and 20 mg/kg QW/Q2W expansion cohorts, respectively. At data cutoff, 13 patients were still responding, of whom 3 have been in response for >28 months.
IMWG response overall and by dose: efficacy evaluable population. sCR, stringent complete response.
IMWG response overall and by dose: efficacy evaluable population. sCR, stringent complete response.
Response rate by previous anticancer therapy, including response by prior regimen or number of prior lines of treatment: efficacy evaluable population. No., number; rel, relapsed; refr, refractory.
Response rate by previous anticancer therapy, including response by prior regimen or number of prior lines of treatment: efficacy evaluable population. No., number; rel, relapsed; refr, refractory.
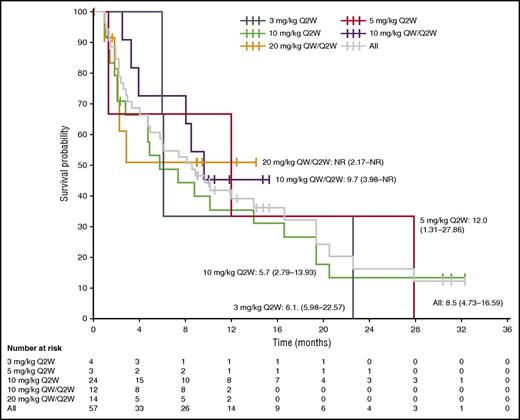
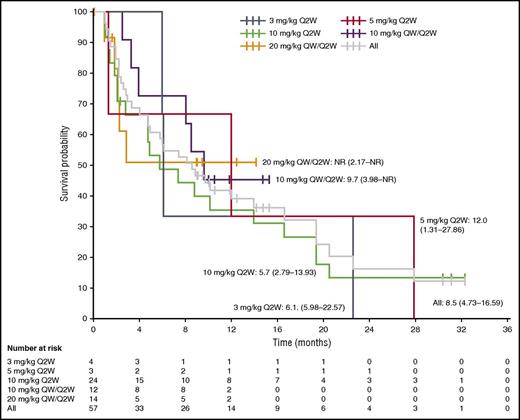
At data cutoff, 37 patients (65%) from the all-treated population had a reported PFS event, and the overall median PFS was 8.5 months (95% confidence interval [CI] 4.73-16.59 months) (Figure 4). Across the total population, the PFS rate at 12 months was 38.9% (95% CI 24.9% to 52.9%). In patients who received isatuximab 10 mg/kg, the PFS was longer with the QW/Q2W than the Q2W schedule (9.7 months vs 5.7 months, respectively); the median PFS was not reached with isatuximab 20 mg/kg QW/Q2W.
Kaplan-Meier analysis of PFS. Median PFS (95% CI) (months) is plotted for each dose cohort and the total population. NR, not reached.
Kaplan-Meier analysis of PFS. Median PFS (95% CI) (months) is plotted for each dose cohort and the total population. NR, not reached.
PK
Evaluation of isatuximab PK (n = 34) after the first administration showed moderate interpatient variability of exposure within each dose level (maximum observed plasma concentration coefficient of variation [CV] = 24% to 52%; area under the plasma concentration vs time curve [AUC] CV = 27% to 55%). Isatuximab AUC0-2wk increased in a greater than dose-proportional manner over the Q2W dose range investigated (4.2-fold increase in exposure with a 3.3-fold increase in dose), suggesting the presence of target-mediated drug disposition with isatuximab (Table 3).
LEN PK parameters at the 25-mg dose were determined for 43 patients, including 29 patients on day 1 and 14 patients on day 15 (Table 3). The geometric mean AUC0-24h for LEN in combination with isatuximab and Dex was comparable after single administration (2370 ng·h/mL; CV = 54%) and multiple dosing (2720 ng·h/mL; CV = 44%). The geometric mean AUC ratio was 1.15, indicating no accumulation of LEN between days 1 and 15.
Discussion
The main goal of this study was to assess the safety of isatuximab in combination with standard doses of LEN-Dex in patients with RRMM. Across the 2 schedules tested (Q2W and QW/Q2W), a single DLT was observed with isatuximab 20 mg/kg, and the MTD for this combination was not reached. The AEs attributable to isatuximab were primarily IARs, which were manageable, predominantly grade ≤2 in severity, and mostly occurred during the first infusion; no IARs were reported after the fourth infusion. The characteristics of the IARs observed in this study are consistent with the notion that infusion and hypersensitivity reactions to mAbs tend to occur during the first or second infusions,27 as has been observed in an isatuximab monotherapy study20 and also in studies of DARA.15,-17 The incidence and severity of IARs appeared greater with the 250 mg/h vs 175 mg/h initial infusion rate, although it should be noted that this was not a prespecified endpoint, and only 18 of 57 patients (32%) received isatuximab at the higher initial infusion rate. However, these data were taken to support the use of this slower initial rate in future studies. In this study, the majority of patients received isatuximab at the slower initial infusion rate of 175 mg/h, yet the median infusion duration was still <5 hours at 20 mg/kg and just >3 hours at 10 mg/kg for the first infusion, and just >4.5 hours and just <2.5 hours for subsequent infusions, respectively.
Neutropenia was the most common grade 3/4 laboratory abnormality, whereas the most common grade 3/4 AEs included infections (pneumonia and lung infection), fatigue, and febrile neutropenia. Notably, neutropenia and infection have also been associated with LEN therapy in previous studies of advanced MM.28,-30 In this study, grade 4 neutropenia and neutropenic complications were more common with isatuximab 10 mg/kg Q2W than in either of the QW/Q2W cohorts. Grade 3/4 thrombocytopenia occurred in approximately one-third of patients, a rate similar to that reported in previous studies of LEN therapy in advanced MM. Of the thromboembolic events that have previously been associated with LEN,30 1 case of grade 1/2 deep vein thrombosis and 1 case of grade 3 pulmonary embolism were reported in this study, where all patients received thromboembolic prophylaxis. Overall, no important drug-related treatment-emergent AEs were observed in the current trial beyond those anticipated based on previous studies of LEN-Dex alone.28,-30
In this study, the combination of isatuximab-LEN-Dex demonstrated encouraging clinical activity, with an ORR of 56% and a CBR of 71% in the efficacy-evaluable population. At data cutoff, the median PFS was 8.5 months and the median duration of response across all groups was 10.9 months. Although this study was not powered to compare dose groups, the ORR was generally similar between the 10 and 20 mg/kg cohorts. These efficacy data are particularly encouraging because of the heavily pretreated nature of this population, with a median of 5 prior lines of therapy and >80% of patients refractory to LEN. Indeed, it is notable that the ORR in this study is broadly similar to that observed in a phase 3 trial of LEN-Dex alone administered to relapsed LEN-naive patients (ORR 60%-61%).31,32
Recently, several phase 3 studies comparing 3-drug combinations against standard LEN-Dex as salvage therapy in RRMM have reported significantly improved activity with the triplet regimens. The ORR and PFS for triplets, including CAR plus LEN-Dex (ORR 87.1%, PFS 26.3 months), ixazomib plus LEN-Dex (ORR 78%, PFS 20.6 months), ELO plus LEN-Dex (ORR 79%, PFS 19.4 months), and notably DARA plus LEN-Dex (ORR 92.9%, PFS 83% at 1 year),8,14,17,33 are impressive. However, these phase 3 studies all included early-relapse patients with MM (ie, having received 1-3 prior lines of therapy), with LEN-naive or -sensitive disease, and very few were refractory to their immediate prior line of therapy. The ORR was 66% to 76% in the doublet control arms (LEN naive/sensitive) of these studies compared with an ORR of 30% with POM and Dex treatment of a LEN-refractory RRMM population.34 For the ELO and DARA triplet arms, the data were comparable to those reported in phase 1/2 single-arm studies, which also included patients with predominantly LEN-naive35 or LEN-sensitive disease.36 As discussed, >80% of patients in our study were LEN refractory, for whom the ORR with isatuximab-LEN-Dex was 52%. Only 8 patients included in the isatuximab-LEN-Dex study were less heavily pretreated (1-2 prior lines of therapy), and the ORR in this subgroup was 100%, comparing more favorably to the phase 3 study results discussed above. The data from this study, together with the trial of DARA plus LEN-Dex in early-relapse patients,17 suggest that potent, synergistic proimmune activity can be achieved by the addition of an IMiD to anti-CD38 therapy across the spectrum of patients with RRMM. Indeed, the ORR of 30% in patients refractory to CAR and POM in this study supports the anti-MM effects of this combination, especially in patients who have limited options for other treatments. Furthermore, isatuximab-LEN-Dex led to clinical responses in a proportion of patients with adverse cytogenetics. Although the patient numbers are low in the current study, these data support a subgroup analysis from the phase 3 POLLUX study of DARA-LEN-Dex for the activity of an anti-CD38 mAb and IMiD combination in this patient population.37
PK assessment revealed that isatuximab behavior in combination with LEN-Dex was consistent with that observed with isatuximab monotherapy. Isatuximab PK was nonlinear in the dose range investigated, and exposure parameters showed moderate interpatient variability but were generally comparable to those reported in a phase 1 monotherapy study.38 Thus, data from the combination and monotherapy studies suggest that the PK parameters of isatuximab were unaltered by LEN administration, with a geometric mean AUC0-2wk ratio (isatuximab-LEN-Dex/isatuximab monotherapy) of 1.04 for the 10-mg/kg dose. When given with isatuximab, LEN exposure (25-mg dose) showed no accumulation after repeat dosing and was within the range reported for LEN monotherapy in patients with MM in the Food and Drug Administration approval package (geometric mean of AUC0-24h 2230 ng·h/mL),39 suggesting no effect of isatuximab coadministration on LEN PK.
In conclusion, the results of this ongoing phase 1b study demonstrate that isatuximab in combination with standard doses of LEN and Dex is well tolerated and active in patients with RRMM. Only 1 DLT was observed, and the MTD was not reached. Objective responses were observed in this heavily pretreated patient population who had relapsed and/or were refractory to currently available PIs and IMiDs, with at least PR attained in over half of the patients who were refractory to LEN before study entry. For this combination, the selected isatuximab dose for further investigation will be 10 mg/kg QW/Q2W.40 This dose is also being tested in a phase 3 trial of isatuximab combination therapy (isatuximab 10 mg/kg QW/Q2W plus POM and Dex in RRMM) that has now been initiated (NCT02990338).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
LEN was provided by Celgene Corporation. The authors received editorial and writing support from Neil Harrison of Adelphi Communications Ltd, funded by Sanofi. The authors thank Yun Ding and Lei Gao of Sanofi for their help as study programmer and statistician, respectively, and Helene Guillemin-Paveau for her involvement in the PK analysis.
This research was funded by Sanofi and in part through the National Cancer Institute, National Institutes of Health Cancer Center Support (grants P30CA008748 [N.L.] and P30CA082103 [T.M.]).
Sanofi collaborated with the authors in the study design, collection, analysis, and interpretation of data and was involved in drafting and reviewing the article.
Authorship
Contribution: T.M., R.B., D.M.B., N.L., J.W., P.M., A.M.L., and R.V. were involved in study design, data collection, data interpretation, and writing of the manuscript; and C.W., E.C., and F.C. were involved in study design, data analysis, data interpretation, and writing of the manuscript. The paper was reviewed and approved by all authors, and the corresponding author had the final responsibility over the decision to submit for publication.
Conflict-of-interest disclosure: T.M. and D.M.B. receive research funding from Sanofi. R.B. receives research funding from Sanofi, Celgene, Millennium, Bristol-Myers Squibb, Karyopharm, and Novartis. J.W. acts as a consultant for Onyx/Amgen, Takeda, and Celgene. P.M. receives research funding from Sanofi and Celgene. A.M.L. receives research funding from Bristol-Myers Squibb, Janssen, and Genentech. C.W., E.C., and F.C. are all employees of Sanofi. R.V. receives research funding from Takeda and Onyx, and honoraria from Onyx, Takeda, Celgene, Bristol-Myers Squibb, Novartis, Janssen, and Merck. N.L. declares no competing financial interests.
Correspondence: Thomas Martin, UCSF Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco Medical Center, 400 Parnassus Ave, San Francisco, CA 94143; e-mail: tmartin@medicine.ucsf.edu.