To the editor:
Sickle cell trait (SCT), defined as heterozygosity for the sickle cell β-globin gene mutation (HbAS), is present in ∼8% to 12% of African Americans.1,2 Despite a lack of longitudinal studies of individuals with SCT, it is generally thought that these heterozygous carriers exhibit a normal lifespan.3 On the other hand, SCT has been associated with an increased rate of venous thromboembolism, ischemic stroke, and renal dysfunction.4-6 It has also been associated with exertional sudden death,7 but a very recent study has challenged this finding when reporting a strong association with rhabdomyolysis.8 Several studies of patients with sickle cell disease (SCD) have shown an association with abnormalities of cardiac structure and function. In particular, an elevated tricuspid regurgitant velocity (TRV) is associated with early mortality in SCD,9-11 whereas another study reported a significant association between SCD and myocardial ischemia.12 The association of SCT with cardiac dysfunction and incident heart failure (HF) has not been comprehensively studied. We examined the association of SCT with (1) risk of HF and (2) abnormalities of cardiac structure and function.
To do this, we performed a meta-analysis of 4 different US population-based cohorts. A brief description of the study methods is given, with a full description of each cohort plus genotyping and quality control methods presented in the supplemental Methods, available on the Blood Web site.
The analysis was performed using African American participants from the following cohorts: Atherosclerosis Risk in Communities Study (ARIC); Jackson Heart Study (JHS); Multi-Ethnic Study of Atherosclerosis (MESA); and the Women's Health Initiative (WHI). The design and methods of each study have been published.13-16 Baseline clinical information was collected by in-person examination and self-report. Data on African American participants between 1987 and 2011 were used for analysis in ARIC, between 2000 and 2013 for JHS, between 2002 and 2012 for MESA, and between 1998 and 2012 for WHI.
The exposure variable was the rs334 single nucleotide polymorphism that produces the HBB p.Glu7Val substitution of hemoglobin S (HbS) or sickle hemoglobin. Assessment of SCT status was performed by direct genotyping, whole exome sequencing, or imputation of rs334 genotypes from genome-wide single nucleotide polymorphism (SNP) genotyping data (Affymetrix 6.0). Individuals who were genotyped or imputed as homozygous for rs334 (based on an allelic dose of >1.5; n = 8; 3 from ARIC, 3 from JHS, and 2 from WHI) or were compound heterozygous such as HbSC were excluded from further analyses.
The main outcome variable was incident HF, and cases were identified and adjudicated from annual follow-up telephone calls to participants, hospital record review, and vital status as previously described.17-19 Further details can be found in the supplemental Methods. Participants with a history of HF at baseline were excluded from the analysis.
To determine changes in cardiac structure and function, echocardiograms were obtained among all consenting JHS participants at visit 1 and consenting ARIC participants at visit 5; thus, mean cohort age at echocardiogram for ARIC subject was about 20 years higher than presented in Table 1. Images were obtained in standard views. Primary measures of left ventricular (LV) dimensions, volumes, and wall thickness; right ventricular (RV) area; left atrial dimension, volume, and area; and Doppler measures of mitral inflow, tricuspid regurgitation, and mitral annular relaxation velocities were made in triplicate from the 2-dimensional views in accordance with the recommendations of the American Society of Echocardiography.20,21 Adverse remodeling was defined as concentric remodeling (relative wall thickness [RWT] >0.42 and no LV hypertrophy (LVH)]; concentric hypertrophy (RWT <0.42 and LVH); or eccentric hypertrophy (RWT ≥0.42 and LVH). Diastolic dysfunction was defined using an approach used previously in a similar community study.22
Categorical variables were compared using χ2 or Fisher’s exact test, and continuous variables were compared using t test or Wilcoxon signed rank test depending on distribution of data. Cox regression was used to calculate hazard ratios for incident HF, adjusting for age, sex, and genetic ancestry. Meta-analysis of hazard ratios (HRs) from each study was performed using a fixed effects model. Between-study heterogeneity was assessed using I2.
After excluding participants with missing data for SCT or clinical covariates and those with HbSS (sickle cell disease) or HbSC, the analysis included 15 364 African Americans (1211 individuals with SCT; 14 153 individuals without SCT). Baseline characteristics of the participants are listed in Table 1. The prevalence of SCT among the cohorts ranged from 7% to 9%, consistent with population prevalence estimates in African Americans.1,2
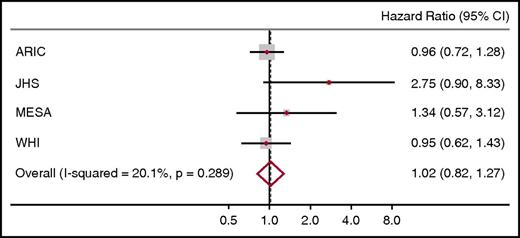
Incident HF (adjudicated using a standard definition) was observed in 1307 participants (92 of 1211 SCT carriers [7.6%] vs 1215 of 14 153 noncarriers [8.6%]). As shown in Figure 1, there was no increased risk of HF in SCT carriers compared with noncarriers (HR, 1.02; 95% confidence interval, 0.82-1.27). The smaller cohorts (MESA and JHS) displayed wider confidence intervals for the HR estimates compared with the larger cohorts (ARIC and WHI), likely reflecting differences in sample size. There were no significant differences in CV risk factors between SCT carriers and noncarriers in any cohort, and all cohorts used similar criteria for adjudicating HF, minimizing potential differences resulting from outcome ascertainment (supplemental Methods).
Meta-analysis of the HRs for HF comparing African American SCT carriers with noncarriers in MESA, WHI, JHS, and ARIC. Each cohort estimate is adjusted for age, sex, and ancestry. The size of data markers indicates the weight of the respective study.
Meta-analysis of the HRs for HF comparing African American SCT carriers with noncarriers in MESA, WHI, JHS, and ARIC. Each cohort estimate is adjusted for age, sex, and ancestry. The size of data markers indicates the weight of the respective study.
The results of selected parameters of cardiac structure and function are presented in Table 2. We found no difference in LV size, wall thickness, or systolic function between SCT carriers and noncarriers (all P > .05). Additionally, TRV as well as RV size and function did not vary according to SCT carrier status. There was also no statistically significant difference in prevalence of adverse remodeling (63% vs 58%, P = .36) or diastolic dysfunction (48% vs 39%, P = .91) between SCT carriers and noncarriers.
We conclude that the presence of SCT was not associated with an increased risk of incident HF or alterations in cardiac structure or function.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank the other investigators, staff, and participants of the Atherosclerosis Risk in Communities (ARIC) study, the Multi-Ethnic Study of Atherosclerosis (MESA) study, the Jackson Heart Study (JHS), and the Women’s Health Initiative (WHI).
The ARIC study is a collaborative study supported by the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C) and NIH/NHLBI grant 5K12 HL087097-0. MESA and the MESA SNP Health Association Resource (SHARe) projects are conducted and supported by the NIH/NHLBI in collaboration with MESA investigators; MESA is supported by grants from the NIH/NHLBI (HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-001079, UL1-TR-000040) and NIH, National Institute of Diabetes and Digestive and Kidney Diseases (DK063491). The JHS is supported by grants from the NIH/NHLBI and the National Institute on Minority Health and Health Disparities (HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, and HSN268201300050C). The WHI program is funded by the NIH, NIH/NHLBI, and US Department of Health and Human Services through contracts (HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C). This work is supported by grants from NIH/NHLBI (3U01 HL117721 02S2) and an Emory Pediatrics pilot grant (00051285) (H.I.H.); NIH/NHLBI (UO1 HL117659) and the Doris Duke Charitable Foundation (2013123) (N.S.K.); and the NIH/NHLBI (1K08HL125100) (R.P.N.). Full lists of participating investigators and institutions can be found as follows: MESA (http://www.mesa-nhlbi.org), JHS (https://www.jacksonheartstudy.org/jhsinfo/Home/tabid/36/Default.aspx), and WHI (https://www.whi.org/SitePages/WHI%20Home.aspx).
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the US Department of Health and Human Services.
Contribution: N.A.B., H.I.H., and S.H.K. were responsible for the design of the research, analysis and interpretation of data, and manuscript generation. A.P.R. analyzed the MESA, JHS, and WHI heart failure data, C.L.C. analyzed the WHI heart failure data, S.R.S. assisted in the analysis of the JHS heart failure data, and N.S.R. performed the meta-analysis. N.A.B. and S.R.S. performed the statistical analysis of the echocardiographic data. All authors contributed to the interpretation of data as well as the critical review and revision of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Natalie A. Bello, Columbia University Medical Center, 177 Fort Washington Ave, Milstein Hospital Building, Echo Laboratory, MHB2-035, New York, NY 10032; e-mail: nb338@cumc.columbia.edu.
References
Author notes
N.A.B. and H.I.H. are joint first authors.