Abstract
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase physiologically expressed by fetal neural cells. However, aberrantly expressed ALK is involved in the pathogenesis of diverse malignancies, including distinct types of lymphoma, lung carcinoma, and neuroblastoma. The aberrant ALK expression in nonneural cells results from chromosomal translocations that create novel fusion proteins. These protein hybrids compose the proximal part of a partner gene, including its promoter region, and the distal part of ALK, including the coding sequence for the entire kinase domain. ALK was first identified in a subset of T-cell lymphomas with anaplastic large cell lymphoma (ALCL) morphology (ALK+ ALCL), the vast majority of which harbor the well-characterized nucleophosmin (NPM)-ALK fusion protein. NPM-ALK co-opts several intracellular signal transduction pathways, foremost being the STAT3 pathway, normally activated by cytokines from the interleukin-2 (IL-2) family to promote cell proliferation and to inhibit apoptosis. Many genes and proteins modulated by NPM-ALK are also involved in evasion of antitumor immune response, protection from hypoxia, angiogenesis, DNA repair, cell migration and invasiveness, and cell metabolism. In addition, NPM-ALK uses epigenetic silencing mechanisms to downregulate tumor suppressor genes to maintain its own expression. Importantly, NPM-ALK is capable of transforming primary human CD4+ T cells into immortalized cell lines indistinguishable from patient-derived ALK+ ALCL. Preliminary clinical studies indicate that inhibition of NPM-ALK induces long-lasting complete remissions in a large subset of heavily pretreated adult patients and the vast majority of children with high-stage ALK+ ALCL. Combining ALK inhibition with other novel therapeutic modalities should prove even more effective.
Introduction
Anaplastic lymphoma kinase (ALK) was initially discovered as an oncogene in human anaplastic large cell lymphomas (ALCLs).1 Together with LTK and ROS, ALK belongs to the insulin receptor superfamily of cell membrane–spanning receptors that display intrinsic tyrosine kinase activity. ALK encodes a tyrosine kinase receptor whose physiologic expression is largely limited to fetal neural cells. Studies with ALK-knockout zebrafish show defects in neural progenitor cell proliferation, differentiation, and survival.2-4 Most ALCLs in children and young adults are ALK positive (ALK+), and the majority of these contain a t(2;5) chromosomal translocation. Although ALCL cells are believed to correspond to mature CD4+ T lymphocytes, recent studies suggest that the ALK gene translocation occurs in an immature thymic precursor cell with stem-like properties.5,6 The t(2;5) translocation fuses a distal part of the ALK gene on chromosome 2p23 with the promoter and a proximal domain of the nucleophosmin (NPM1) gene on chromosome 5q35. NPM is a ubiquitously expressed nucleolar protein that is involved in ribosomal component shuttling between the cytoplasm and nucleus.7,8 This translocation yields an 80-kD NPM-ALK chimeric protein that contains the oligomerization motif of NPM fused to the cytoplasmic portion of ALK with an intact kinase domain.1,2,9 The NPM-ALK protein is expressed as a homodimer, which becomes autophosphorylated through reciprocal ALK tyrosine kinase activity and, consequently, it is strongly and persistently activated.10,11 At least 14 other ALK translocation partners are recognized in ALCL and other tumors.12 These malignancies include subsets of large B-cell lymphoma,13 the distinct adenocarcinoma subtype of non–small-cell lung carcinoma (NSCLC),14 rhabdomyosarcoma, inflammatory myofibroblastic tumor, sickle cell trait–associated renal medullary carcinoma,12 papillary thyroid carcinoma,15 cutaneous Spitz nevi and spitzoid melanomas,16 and neuroblastoma.17,18 Although some ALK fusion proteins are generally shared by these histologically diverse tumors, the predominant translocations such as NPM-ALK in ALCL and EML4-ALK in NSCLC are very characteristic for their respective tumor types. NPM-ALK serves as the founding member of the ALK fusion protein family, and its role in malignant cell transformation is by far the best characterized and, thus, is the main focus of this review.
NPM-ALK hijacks cell signaling pathways physiologically used by the interleukin-2 cytokine family
As a persistently active tyrosine kinase, NPM-ALK activates multiple intracellular signal transduction pathways. These pathways include PLCγ, PI3K-AKT, MAPK/ERK, mTOR, STAT3, and STAT5b; they have been reviewed in detail before,19 and together with their target genes, are schematically depicted in Figure 1. Of note, comparison of ALK-dependent and interleukin-2 (IL-2) –dependent signaling in T-cell lymphoma cell lines showed greatly overlapping gene expression profiles enriched in genes regulated by JAK-STAT– and IL-2–dependent pathways.20 Given that IL-2 and similar cytokines such as IL-7 and IL-21 are required for normal T-cell differentiation and proliferation, this study suggests that NPM-ALK mimics physiological progrowth signals and provides tumor cells with the necessary cues to secure their survival and expansion (Figure 1).
NPM-ALK signals through the T-cell receptor (TCR), IL-2-type cytokine, and STAT3 pathways. Aberrant NPM-ALK kinase activity hijacks key cell signaling pathways physiologically activated by TCR and IL-2 signaling pathways. Epigenetic silencing of the tumor suppressor genes SHP-1, STAT5a, and IL-2Rg by STAT3-activated DNA methyltransferases (DNMTs) inhibits the downregulation of NPM-ALK. Dashed lines reflect loss of expression and function as a result of epigenetic gene silencing. Transcriptional targets directly downstream of these signaling pathways include key transcription factors and other cell cycle and apoptosis regulators that normally promote growth and survival but lead to oncogenesis in ALK+ ALCL. Other direct transcriptional targets include histologic markers (CD30, CD274), immunosuppressive cytokines (IL10, TGFβ), and angiogenic factors (HIF1α). Phosphoproteomic analysis has revealed that in addition to modulating important signaling pathways, NPM-ALK alters key metabolic pathways through phosphorylation, such as that of the rate-limiting enzyme pyruvate kinase, whose phosphorylation inactivates the enzyme promoting a shift from aerobic to anaerobic glycolysis.
NPM-ALK signals through the T-cell receptor (TCR), IL-2-type cytokine, and STAT3 pathways. Aberrant NPM-ALK kinase activity hijacks key cell signaling pathways physiologically activated by TCR and IL-2 signaling pathways. Epigenetic silencing of the tumor suppressor genes SHP-1, STAT5a, and IL-2Rg by STAT3-activated DNA methyltransferases (DNMTs) inhibits the downregulation of NPM-ALK. Dashed lines reflect loss of expression and function as a result of epigenetic gene silencing. Transcriptional targets directly downstream of these signaling pathways include key transcription factors and other cell cycle and apoptosis regulators that normally promote growth and survival but lead to oncogenesis in ALK+ ALCL. Other direct transcriptional targets include histologic markers (CD30, CD274), immunosuppressive cytokines (IL10, TGFβ), and angiogenic factors (HIF1α). Phosphoproteomic analysis has revealed that in addition to modulating important signaling pathways, NPM-ALK alters key metabolic pathways through phosphorylation, such as that of the rate-limiting enzyme pyruvate kinase, whose phosphorylation inactivates the enzyme promoting a shift from aerobic to anaerobic glycolysis.
STAT3 has emerged as a critical mediator of NPM-ALK–induced tumorigenesis.21 In fact, STAT3 upregulation seems to be a common feature of ALCL regardless of ALK expression,22,23 because mutational genomic DNA and RNA expression analysis of ALK-negative (ALK–) ALCL revealed STAT3 upregulation by other tyrosine kinase chimeras containing either ROS, a close cousin of ALK, or TYK2, a member of the JAK family. Moreover, activating point mutations of JAK1 and/or STAT3 itself were also found in the ALK– ALCL patients.22 These observations provide further evidence for ALK/ROS mimicry of IL-2/IL-7/IL-21 signaling in the malignant transformation of the CD4+ T cell and for the central role of STAT3 activation in the pathogenesis of the ensuing ALCL. Importantly, these findings also suggest that patients with either ALK+ or ALK– ALCL might benefit from therapeutically targeting STAT3.
Expression and pathogenic role of microRNAs in ALK+ ALCL
Several groups have used RNA sequencing methods to identify microRNAs (miRs) that may contribute to the pathogenesis of ALK+ ALCL.24-26 Nine miRs are preferentially expressed in ALK+ ALCL cells, including miR-155, the miR also expressed in subsets of ALK– ALCL and B-cell lymphomas,26 as well as activated T and B cells,26 suggesting its cell growth–promoting function. In contrast, downregulation of miR-101 seems necessary for NPM-ALK–induced oncogenesis, because its forced expression attenuated cell growth.27 The miR-17-92 cluster, reported as upregulated by both MYC28 and STAT3,29 contributes to proliferation and survival of ALK+ ALCL cells.29 Furthermore, iNOS expression is upregulated through STAT3-induced inhibition of an iNOS inhibitor, miR-26a.30 Altered miR expression downstream of STAT3 was also found to inhibit apoptosis through suppression of BIM expression.29 Finally, miRs have an impact on other features of ALK+ ALCL, including the IL-17 T-cell phenotype,31 production of the angiogenic factor VEGF,32 and inhibition of apoptosis by other mechanisms.33
In summary, a number of functionally diverse miRs are aberrantly expressed in ALK+ ALCL with expression of some controlled by NPM-ALK. They seem to play a significant role in the NPM-ALK–induced malignant cell transformation by affecting many key functional properties of the ALK+ ALCL cells. Consequently, miRs may become attractive therapeutic targets in ALK+ ALCL and similar malignancies.
Functional consequences of NPM-ALK activation
Although the role of NPM-ALK in cell proliferation and survival is well established, it has become clear that this chimeric kinase induces several other pro-oncogenic mechanisms through modulation of gene expression, primarily via STAT3, or by changing the functional status of proteins through phosphorylation.
Evasion of anti-tumor immune response.
NPM-ALK promotes immune evasion by inducing both soluble immunosuppressive cytokines and cell membrane immune checkpoint proteins. Accordingly, NPM-ALK acts through STAT3 to induce expression of transforming growth factor beta (TGF-β) and IL-10 as well as the cell-surface receptor PD-L1 (CD274, B7-H1) on the tumor cells34-37 and thus creates a highly immunosuppressive tumor microenvironment. The NPM-ALK-STAT3 pathway also induces expression of ICOS, a member of the CD28 costimulatory receptor superfamily.38 Whereas ICOS engagement stimulates proliferation of ALK+ ALCL cells, it can be argued that by absorbing its ligand (ICOS-L), tumor-expressed ICOS deprives the immune cells of this important activating factor and thus further impairs the antilymphoma response. Of interest, STAT3 promotes ICOS expression not only by directly activating its gene via direct binding to its promoter but also by protecting ICOS messenger RNA (mRNA) through inhibition of miR-219 expression.38 Furthermore, ALK+ ALCL cells fail to express another immunomodulatory molecule, TNFα, at least in part as a result of methylation of its gene’s promoter.39 They do, however, uniformly express the type 1 tumor necrosis factor alpha (TNF-α) receptor (TNFR1) known to transduce proapoptotic signals. Indeed, treatment with TNF-α inhibited growth of ALK+ ALCL cells and induced their apoptotic death, indicating that inhibition of TNFα expression by the malignant cells is yet another mechanism to ensure their survival. Finally, the existing evidence that anti-NPM-ALK antibody serum concentration inversely correlates with disease prognosis suggests that the NPM-ALK protein itself is immunogenic and that immune responses against the NPM-ALK–expressing tumor at least partly control the rate of disease progression.40 Indeed, ALK+ ALCL patients harbor B- and T-lymphocyte populations specific for ALK-encoded epitopes,41,42 although their effectiveness and relative contributions to the immune response to ALK+ ALCL remain poorly defined.
In summary, the above-cited findings reveal that, despite the emergence of anti-tumor response, NPM-ALK–expressing cells are able to suppress the immune system by diverse mechanisms to evade destruction. Consequently, these insights open up novel therapeutic options aimed at counteracting the NPM-ALK–induced mechanisms of immune-response evasion used by ALK+ ALCL.
Tolerance of hypoxia and induction of angiogenesis.
STAT3 also induces expression of HIF1α through direct binding to the HIF1α promoter43 permitting ALK+ ALCL cells to adapt to hypoxic conditions that typically emerge focally in fast-growing tumors. HIF1α contributes to tumor angiogenesis by inducing expression of VEGF.43,44 Of interest, ALK collaborates with HIF1α to boost VEGF expression by down-regulating miR-16, an inhibitor of VEGF mRNA.32
Silencing of tumor suppressor genes.
NPM-ALK–activated STAT3 also has a profound inhibitory effect on gene expression by recruiting to gene promoters the epigenetic gene-silencing complex that contains DNA methyltransferases (DNMTs) 1, 3a, and 3b and histone deacetylase 1.45-49 This NPM-ALK-STAT3–induced epigenetic gene silencing has been shown for SHP-1,45-47 STAT5a,48 and IL2Rγ.49 Of note, protein products of these genes act in ALK+ ALCL cells as tumor suppressors by interfering with expression of NPM-ALK (Figure 1). miR-150 emerged as another tumor suppressor candidate epigenetically silenced by the NPM-ALK-STAT3 axis,50 although the exact mechanisms of its anti-tumor activity remain to be elucidated.
Silencing of T-cell receptor complex.
NPM-ALK has also been reported to epigenetically silence several key genes from the TCR complex and its signaling pathway, including CD3E, ZAP70, LAT, and SLP76 (Figure 1).51 Although the functional consequences of re-expression of any of these genes has not been evaluated, the silencing likely contributes in a significant manner to the substantial loss of the T-cell lineage identity seen in ALK+ ALCL.
DNA repair.
NPM-ALK has been found to affect activity of selected mismatch repair (MMR) proteins. NPM-ALK binds the MMR protein MSH2 but not its partners MSH3 or MSH6,52 and consequently impairs MSH2:MSH6 heterodimerization and function. The MMR function could be restored by disrupting NPM-ALK binding to MSH2.53 NPM-ALK exerts its inhibitory effect by phosphorylating MSH2 at tyrosine 238 (Y238), and introduction of the Y238F mutant leads to marked restoration of the MMR function.54 These findings indicate that NPM-ALK induces genome-wide destabilization by interfering with DNA damage repair processes, which permits the accumulation of additional genetic changes.
Tissue invasiveness and tumor spread through induction of a stem cell–like program.
NPM-ALK taps into another oncogenic mechanism to enhance cell proliferation, invasiveness, and metastatic spread: reversion to a more undifferentiated phenotype through the expression of embryonic genes such as SOX2.55 SOX2 is a known STAT3 target in embryonic and neural stem cells56 and is also a direct target, along with the other key stem cell transcription factors OCT4 and NANOG, of NPM1.57 SOX2 is expressed by ALK+ ALCL cell lines and primary tumors,51 and its expression is enriched in a subpopulation of cells with stem cell–like properties on the basis of side population analysis.5 The protein is functionally active only in a subset of the malignant cells, and SOX2-active cells exhibit better invasiveness and tumorigenesis; short hairpin RNA–mediated depletion of SOX2 affects cell growth and apoptosis in SOX2-active but not SOX2-inactive cells.57
Another embryonic-restricted gene, SALL4, is also upregulated in ALK+ ALCL.58 Similar to SOX2, ALK+ ALCL cell lines contain cells with high and low activity of SALL4 with cells high in SALL4 being functionally more impaired in response to short hairpin RNA–mediated SALL4 depletion. Another stem cell–ssociated gene, TWIST1, was identified in ALK+ ALCL cell lines and tissues.59 TWIST1 is highly expressed in hematopoietic stem cells and promotes their self-renewal and stemness.60 It also contributes to epithelial-mesenchymal transition61 and tumor cell tissue invasion and metastasis formation in gastric,62 breast,63 and hepatocellular64 carcinomas. TWIST1 promotes invasiveness of ALK+ ALCL cells, its expression is NPM-ALK– and STAT3-dependent, and its knockdown sensitizes the cells to ALK inhibition.59
NPM-ALK–promoted activation of cell metabolism.
In a proteomic screen to identify the targets of NPM-ALK kinase activity, more than 600 proteins exhibited altered and almost exclusively decreased phosphorylation in response to an ALK inhibitor.65 One of these NPM-ALK targets is pyruvate kinase PKM2; its inhibition by NPM-ALK–mediated phosphorylation results in a shift from oxidative phosphorylation to anaerobic glycolysis (Figure 1), as well as enhanced cell proliferation in vitro and tumorigenesis in vivo. A small molecule activator of PKM2 suppressed cell growth, suggesting that the kinase could become yet another therapeutic target in ALK+ ALCL. Another phosphoproteomic analysis of cells with NPM-ALK expression identified targets in TNF family–related apoptotic and metabolic pathways.66 Proteomic analysis of Jurkat cells expressing NPM-ALK identified increased expression of proteins involved in signaling pathways downstream of NPM-ALK, including STAT3.67 A different proteomic screen identified 254 NPM-ALK binding partners involved in wide-ranging biological processes, including metabolic pathways.52 We recently found that NPM-ALK induces expression of NAMPT, the key rate-limiting enzyme in the NAD+ synthesis pathway (Q.Z., H. Wang, X. Liu, S. Chekol, L. Guo, A. Ziober, M. Feldman, J. Svoboda, J. D. Glicskon, S. D. Turner, I. A. Blair, C. Van Dang, S. J. Schuster, M.A.W., manuscript in preparation). Highly specific NAMPT inhibitors are available and may prove highly effective in ALK+ ALCL therapy.
NPM-ALK–mediated transformation of human CD4+ T cells
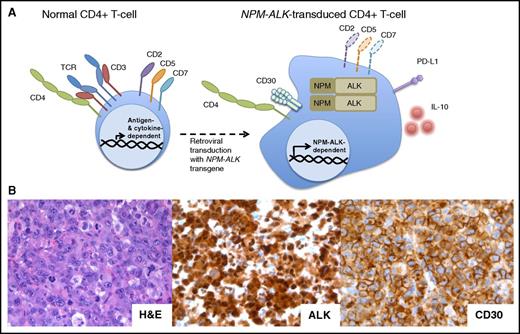
The mechanisms elaborated above detail how constitutive NPM-ALK activation induces protein phosphorylation cascades that result in global changes in gene expression and protein activity and profound modulation of key cell functions. These potent oncogenic features of NPM-ALK were recently highlighted in a dramatic way by demonstrating that this fusion kinase is able to immortalize in vitro normal human CD4+ T lymphocytes (Figure 2).68 The resultant cell lines share the distinct morphologic and immunophenotypic features with patient-derived ALK+ ALCL. When xenotransplanted into mice, they produce tumors histologically indistinguishable from native ALK+ ALCL. Interestingly, TCR analysis indicated that these cell lines were mono- or oligoclonal, suggesting that only a subset of peripheral human CD4+ T cells are susceptible to ALK-mediated transformation. Of note, depending on the promoter used, NPM-ALK transgenic mice largely produce B-cell lymphomas or T-cell tumors that correspond to immature T-cell lymphoblastic lymphoma rather than ALCL.69,70 These findings indicate that the NPM-ALK–mediated transformation of human CD4+ T cells may prove superior to the NPM-ALK transgenic mouse models for studying ALK+ ALCL. The oligo-/monoclonal nature of the tumors emerging after the NPM-ALK gene transduction in human CD4+ T cells suggests the possible need for additional genetic changes to achieve malignant phenotype. It may be relevant in this context that the presence of NPM-ALK transcript has been reported in healthy newborns.71
The NPM-ALK transgene can transform normal human CD4+peripheral T-cells into ALCL. (A) NPM-ALK–transduced normal CD4+ peripheral T cells become immortalized and acquire morphology and surface phenotype of ALCL, including expression of CD30, PD-L1, and IL-10, at least partial loss of the TCR, CD2, CD5, and CD5, and maintenance of CD4. (B) Histologic examination of the tumors formed by the NPM-ALK–transformed CD4+ T cells xenotransplanted into nonobese diabetic/severe combined immunodeficient γ mice reveals their striking and immunophenotypic resemblance to the primary, patient-derived ALK+ ALCL. H&E, hematoxylin and eosin.
The NPM-ALK transgene can transform normal human CD4+peripheral T-cells into ALCL. (A) NPM-ALK–transduced normal CD4+ peripheral T cells become immortalized and acquire morphology and surface phenotype of ALCL, including expression of CD30, PD-L1, and IL-10, at least partial loss of the TCR, CD2, CD5, and CD5, and maintenance of CD4. (B) Histologic examination of the tumors formed by the NPM-ALK–transformed CD4+ T cells xenotransplanted into nonobese diabetic/severe combined immunodeficient γ mice reveals their striking and immunophenotypic resemblance to the primary, patient-derived ALK+ ALCL. H&E, hematoxylin and eosin.
NPM-ALK as the ultmate oncogene
Hanahan and Weinberg72 proposed an elegant conceptual framework termed the “Hallmarks of Cancer” to understand the multiple mechanisms by which a normal cell becomes a malignant cell. The original pillars included self-sufficiency in growth signals, insensitivity to antigrowth signals, tissue invasion and metastasis, limitless replicative potential, sustained angiogenesis, and resistance to apoptosis. When the authors revisited the framework a decade later, they added the pillars of deregulated cellular energetics, evasion of immune-mediated destruction, tumor promoting inflammation, and mutagenic genome instability.73 In this review, we have summarized how NPM-ALK, a chimeric tyrosine kinase, induces most if not all of these oncogenic features (Figure 3).74 Accordingly, NPM-ALK homodimerizes, autophosphorylates, and initiates signaling cascades that recapitulate IL-2–type progrowth cell signaling by modulating expression of protein- and miR-encoding genes, as well as by altering the functional status of intracellular proteins. NPM-ALK also protects cells from hypoxia and induces angiogenesis, deregulates DNA repair pathways, shifts cancer cell metabolism, and induces embryonic transcriptional programs that permit tissue evasion and metastasis.
NPM-ALK achieves most of the “Hallmarks of Cancer.” NPM-ALK is the protein product of a chromosomal translocation. Through activation of T-cell signaling pathways and dysregulation of the phosphoproteome, NPM-ALK drives the transformation of T cells into ALK+ ALCL by multiple oncogenic mechanisms. Diagram modified from Hanahan and Weinberg.73 (*) According to Lai and Ingham.74
NPM-ALK achieves most of the “Hallmarks of Cancer.” NPM-ALK is the protein product of a chromosomal translocation. Through activation of T-cell signaling pathways and dysregulation of the phosphoproteome, NPM-ALK drives the transformation of T cells into ALK+ ALCL by multiple oncogenic mechanisms. Diagram modified from Hanahan and Weinberg.73 (*) According to Lai and Ingham.74
NPM-ALK as the ultimate therapeutic target
On the basis of preclinical and early clinical studies in ALK+ ALCL and other ALK-driven malignancies, the kinase is an extremely attractive therapeutic target and thus several ALK inhibitors are at various stages of clinical testing and US Food and Drug Administration (FDA) approval (Table 1).75 Although compared with other T-cell lymphomas, ALK+ ALCL is highly sensitive to the standard combination chemotherapy with 5-year overall survival (OS) in the range of 70% to 90%, depending on the population studied,76 the long-term toxicities as well as late relapses pose a considerable clinical problem. Whereas most clinical results regarding ALK inhibitors come from patients with ALK+ NSCLC, it is clear that ALK inhibition is effective in all ALK-expressing malignancies evaluated so far and especially so in ALK+ ALCL. Indeed, the first reported administration of crizotinib, a first-generation ALK inhibitor, in 7 adults with therapy-resistant, high-stage ALK+ ALCL resulted in complete response (CR) in 3 patients and partial response in 1 patient.77 The same investigators expanded the study to a total of 11 patients (9 with ALCL and 2 with diffuse large B-cell lymphoma). At the time of the report,78 the response was observed in 10 patients, 9 of whom experienced CR. With a 2-year follow-up, OS was 72.7% and progression-free survival (PFS) was 63.7%, indicating high durability of the response. The preliminary results in the pediatric population are even more encouraging. The Children’s Oncology Group–sponsored phase 1/2 clinical trial (NCT00939770) with crizotinib in children with refractory ALK+ ALCL, solid tumors, or neuroblastoma resulted in CR in 8 of 9 patients with ALK+ ALCL, by far exceeding the response rate in the other ALK-expressing malignancies.79 Although the PFS and OS data are not yet available, it is already clear that the CRs in ALK+ ALCL are highly durable and long lasting.80
However, resistance to ALK inhibitors, including even second- or third-generation drugs used as a single therapy, is all but inevitable in at least a subset of ALK+ ALCL patients. Resistance to crizotinib was originally reported in NSCLC81,82 and inflammatory myofibroblastic tumor,83 followed by neuroblastoma79 and ALCL.78 Mechanisms of resistance fall roughly into 2 categories: (1) inhibitor resistance chiefly as a result of mutations of the ALK gene impairing binding of an inhibitor to ALK protein, and (2) engagement of other cell signaling pathways. In the former scenario, malignant cells remain ALK dependent, but in the latter scenario, they gain ALK independence by using alternative oncogenic mechanisms such as mutations of KRAS or EGFR, as already seen in ALK+ NSCLC.84-86 Structural analysis of the ALK gene in 2 adults with ALK+ ALCL who became nonresponsive to crizotinib revealed mutations in the ALK kinase domain, suggesting that the inhibitor resistance, rather than gain of ALK independence, caused the disease progression in these patients.78
ALK gene mutations are quite diverse and their type may impact clinical decisions. Some mutations, such as the kinase pocket gatekeeper mutation at L1196, which is analogous to the gatekeeper mutations seen in ABL and EGFR kinases,87,88 may be overcome by most of the second- and third-generation drugs, but other mutations are particularly cross-resistant and remain sensitive to only a subset of the available inhibitors. Indeed, clinical studies have shown that most but not all patients with ALK+ NSCLC who lost sensitivity to crizotinib respond to ceritinib or alectinib, 2 drugs now approved by the FDA for therapy in crizotinib-resistant patients.89,90 Similar results have been seen in patients with ALK+ NSCLC who were treated with the other ALK inhibitors listed in Table 1.91-94 In ALK+ ALCL cell lines, resistance to both first-generation crizotinib and second-generation alectinib was a result of the emergence of additional mutations beyond the gatekeeper mutation.95 Some mutations, at I1171 for instance, are particularly cross-resistant.96 It can be argued, therefore, that DNA sequence analysis of the ALK gene in individual patients and matching of the identified mutations to the specific ALK inhibitors active against these mutations should be required to optimize the therapy of patients who have developed resistance to crizotinib. The same principle of an in-depth structural ALK gene analysis should apply to tumors that failed to respond to the second- or third-generation ALK inhibitors. Given the emergence of resistance mechanisms independent of ALK gene mutations, the tumors may need to be evaluated for the presence of these alternative resistance pathways, in particular if no ALK mutations are detected. Of note, a third-generation inhibitor, lorlatinib, remains active against a broad range of known ALK mutations in preclinical models of ALK+ NSCLC and neuroblastoma.97-99 In a recent case study, a patient with ALK+ NSCLC developed resistance to first- and second-generation ALK inhibitors. The patient was subsequently treated with lorlatinib. A malignant cell clone with yet a third resistance mutation emerged; however, it displayed renewed sensitivity to crizotinib,93 suggesting that prospective therapy with a combination of ALK inhibitors with activities against diverse mutants may prove superior to therapy with a single ALK inhibitor. Furthermore, it is conceivable that additional, even more effective ALK inhibitors will be developed. Accordingly, optimization of the crizotinib scaffold based on its cocrystal structure with the ALK kinase domain resulted in a compound with 100-fold increased potency against wild-type ALK and 67- to 825-fold increased activity against various clinically relevant mutants.100
Given the all but inevitable ALK inhibitor resistance, it is more than likely that drug combination therapy targeting not only ALK but also other pro-oncogenic molecules or pathways will be required to achieve durable CRs or cures in the majority of patients with ALK+ ALCL and other ALK-driven malignancies. Accordingly, a phase 4 multicenter clinical trial comparing crizotinib plus chemotherapy with crizotinib alone in adult ALK+ ALCL patients (NCT02487316) is currently underway, and a Children’s Oncology Group phase 1 study to test crizotinib plus chemotherapy in children (NCT01606878) is enrolling patients. Although it is likely that these trials will prove highly successful, given the considerable efficacy of crizotinib alone and standard chemotherapy alone in ALK+ ALCL, there may still be patients for whom treatment will fail, especially among adults with high-stage disease. Furthermore, long-term adverse effects of chemotherapy, such as secondary malignancies, are of particular concern in the pediatric population. Therefore, the search for ALK inhibitor combinations with novel, less toxic agents is warranted. Some of these combinations may contain inhibitors of key cell signaling pathways downstream of NPM-ALK or key effectors of ALK oncogenicity, detailed earlier. For example, ALK inhibition may be combined with inhibition of mTOR because ALK+ ALCL cells are sensitive to mTOR inhibition.101
STAT3 is another attractive candidate, because of the key NPM-ALK–activated gene transcriptional modulator.19,21 However, the currently available STAT3 inhibitors have serious limitations because they are poor tissue-penetrating oligonucleotides or small molecules with rather low specificity.102 Given the role of DNMTs and other members of the epigenetic machinery in inhibiting tumor suppressors in ALK+ ALCL,19,39,45-49 they are also potential therapeutic cotargets with ALK.
Finally, targeting proteins involved in the NPM-ALK–triggered evasion of the immune system, the foremost being PD-L1,35,68 may also prove beneficial, given the clinical efficacy of antibodies blocking the PD-1–PD-L1 axis seen in other malignancies.
Of note, ALK-driven malignancies seem quite sensitive to HSP90 inhibitors, as shown in preclinical models of neuroblastoma,79 NSCLC,103 and ALCL.95 In the latter study, the cell lines remain sensitive to HSP90 and mTOR inhibition after developing resistance to ALK inhibition. A third-generation HSP90 inhibitor, ganetespib, was shown to be well tolerated in a phase 2 trial of advanced-stage NSCLC.104 In that study, 99 patients were enrolled, but only 4 showed a partial response. Of these 4, all tested positive retrospectively for an ALK rearrangement, suggesting that ALK+ tumors may be particularly sensitive to HSP90 treatment, as shown mechanistically in vitro.105 Therefore, a combination of ALK inhibition with inhibition of HSP90 could be explored. Likewise, a recent study indicates that targeting cell autophagy with the clinically available drug chloroquine may further sensitize ALK+ ALCL cells to ALK inhibition.106
Another attractive candidate for combination with ALK inhibition in ALCL is the anti-CD30 agent brentuximab vedotin (Adcetris), a monoclonal antibody fused to a microtubule inhibitor. CD30, a member of the TNF receptor family, is strongly expressed by ALK+ ALCL in an ALK-dependent manner.68 Brentuximab is FDA-approved for a first-line single-agent therapy on the basis of a phase 2 trial and subsequent 3-year follow-up data showing an overall response rate of 86% and CR of 43% in ALCL patients, regardless of the ALK expression status.75,107,108 Even greater therapeutic success was achieved in the ALCL patients when this immunotoxin was combined with chemotherapy, yielding an objective response rate of 100% and CR of 84% with an estimated 3-year PFS of more than 50%.109 In turn, a patient with chemotherapy-refractory ALK+ ALCL displayed profound clinical responses to the consecutive administration of crizotinib and brentuximab.109 When combined, these clinical results strongly suggest that anti-CD30 immunotoxic therapy alone, or together with chemotherapy, should benefit from combination with an ALK inhibitor.
Finally, a preclinical study supported by the therapeutic administration of imatinib in a single patient indicates that targeting PDGF receptor may prove beneficial in ALK+ ALCL,110 such that it too could be a facet of a multipronged approach to this type of lymphoma.
In conclusion, the NPM-ALK chimeric kinase is a very powerful oncogene capable of transforming normal T lymphocytes. By activating a number of key cell signaling and metabolic pathways, with the STAT3 pathway playing the preeminent role, it reprograms the target cells to display classic hallmarks of cancer, including perpetual and invasive growth (in part through epigenetic silencing of tumor suppressor genes and acquisition of stem cell–like properties, respectively), evasion of immune response, adaptation to hypoxia, and dysregulation of DNA repair. Consequently, NPM-ALK is an extremely attractive therapeutic target and the preliminary results with the first-generation ALK inhibitor are very encouraging, in particular in the pediatric population. Combining ALK inhibition with other novel therapies, especially those that target the key effectors of the NPM-ALK–induced malignant cell transformation, may ultimately replace the current standard therapeutic modalities.
Acknowledgments
M.A.W. and Q.Z. have patents issued/pending on “Anaplastic Lymphoma Kinase (ALK) as an Oncogene Capable of Transforming Normal Human Cells” and “Stat5a and Its Functional Tumor Suppressor Analogs for Treatment of Malignancies Expressing NPM/ALK and Other Oncogenic Kinases” assigned to the University of Pennsylvania. M.A.W. has received speaker fees from Seattle Genetics.
Authorship
Contribution: M.T.W. and C.Z. wrote the manuscript; Q.Z. provided key data and edited the manuscript; and M.A.W. wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mariusz A. Wasik, University of Pennsylvania Medical Center, Department of Pathology and Laboratory Medicine, 280 John Morgan Building, Philadelphia, PA 19104; e-mail: wasik@mail.med.upenn.edu.