Abstract
Atypical chronic myeloid leukemia, BCR-ABL1 negative (aCML) is a rare myelodysplastic syndrome (MDS)/myeloproliferative neoplasm (MPN) for which no current standard of care exists. The challenges of aCML relate to its heterogeneous clinical and genetic features, high rate of transformation to acute myeloid leukemia, and historically poor survival. Therefore, allogeneic hematopoietic stem cell transplantation should always be an initial consideration for eligible patients with a suitable donor. Nontransplant approaches for treating aCML have otherwise largely relied on adopting treatment strategies used for MDS and MPN. However, such therapies, including hypomethylating agents, are based on a paucity of data. With an eye toward making a more meaningful impact on response rates and modification of the natural history of the disease, progress will rely on enrollment of patients into clinical trials and molecular profiling of individuals so that opportunities for targeted therapy can be exploited.
Introduction
The current diagnostic criteria that comprise the World Health Organization (WHO) entity “atypical chronic myeloid leukemia, BCR-ABL1 negative” (aCML; Table 1) represent a decades-long evolution of classifying diseases that exhibited morphologic similarity to chronic myeloid leukemia (CML) but lacked both the Ph chromosome by standard cytogenetics and BCR-ABL1 rearrangement by polymerase chain reaction.1,2 The differential diagnosis of these BCR-ABL1-negative hematologic neoplasms not only includes aCML, but also chronic myelomonocytic leukemia (CMML), chronic neutrophilic leukemia (CNL), and myelodysplastic syndrome/myeloproliferative neoplasm, unclassifiable (MDS/MPN, U).1-6
Some cases of aCML have been given the historical moniker “CML-like syndrome” because both diseases exhibit bone marrows with hyperplastic myeloid hyperplasia and peripheral blood leukocytosis characterized by a spectrum of myeloid immaturity. However, on morphologic grounds, this is where the similarity ends. Unlike BCR-ABL1-positive CML, aCML is characterized by prominent dysplastic granulopoiesis (eg, the acquired Pelger-Huët anomaly; nuclear abnormalities including hypersegmentation, nuclear projections, and abnormally clumped nuclear chromatin; and abnormalities of cytoplasmic granules, such as hypogranularity), and in some cases, multlineage dysplasia may be observed.1,2 The finding of ≥10% immature myeloid cells (promyelocytes, myelocytes, and metamyelocytes) in the peripheral blood and/or dysplasia are useful criteria in distinguishing aCML from CNL, which lacks these features.1,2 Additional features of aCML include absent or minimally present basophilia (<2% of leukocytes) and monocytosis (<10% of leukocytes), which are additional morphologic findings that help distinguish aCML from BCR-ABL1-positive CML and CMML, respectively.1,2
The knowledge gleaned from next-generation sequencing has complemented morphologic and laboratory WHO criteria for myeloid neoplasms and can often provide greater specificity in distinguishing aCML from alternative MDS/MPN or MPNs.1,3-7 Invariably, how I pursue the diagnosis and treatment aCML requires attention to the results of standard cytogenetic analysis and myeloid mutation testing because druggable targets may be unmasked.
Case 1
LJ is a 62-year-old woman with a past medical history of hyperlipidemia and a left total hip replacement. In the last 3 months, during 2 episodes of diverticulitis with associated gastrointestinal bleeding requiring red blood cell transfusion support, a new leukocytosis of 15 × 109 to 20 × 109/L was identified. The platelet count was normal, and a manual differential revealed 46% neutrophils, 12% band forms, 12% metamyelocytes, 6% monocytes, 6% myelocytes, 2% promyelocytes, 2% eosinophils, and 14% lymphocytes. The increased WBC count and left-shifted WBC differential was felt to be reactive because of her acute medical condition. The patient was seen in consultation in hematology clinic 2 months after hospital discharge because of persistent blood count abnormalities despite resolution of her gastrointestinal issues. No hepatosplenomegaly was noted on examination. A complete blood count showed persistent elevation of the WBC count to 24.2 × 109/L, hemoglobin of 11.2 g/dL, and platelet count 160 × 109/L with a similar spectrum of myeloid immaturity. A bone marrow aspirate and biopsy revealed hypercellularity for age, left-shifted myeloid hyperplasia without increased blasts, and hypogranular granulocytes with abnormal nuclear segmentation. Dyserythropoiesis and dysplastic megakaryocytes, including hypolobated forms, were noted. Cytogenetics were normal, and polymerase chain reaction for BCR-ABL1 was negative. A diagnosis of aCML was made. The patient was referred for evaluation of management options.
Case 2
CK is a 76-year-old man with a history of coronary artery disease and hepatitis C that had been treated with pegylated-interferon-α-2a (PEG-IFN-α-2a) until 1.5 years ago when it was discontinued because of progressive depressive symptoms and cytopenias. After 4 months off therapy, the patient reported increasing fatigue and new night sweats. A spleen tip was palpated on examination. The WBC count increased from 2.5 × 109 to 26.2 × 109/L after PEG-IFN-α-2a was stopped; the hemoglobin was 10.6 g/dL, and the platelet count was 133 × 109/L. Although an automatic differential showed 89% neutrophils, a manual differential revealed 17% neutrophils, 27% band forms, 6% metamyelocytes, 8% monocytes, 15% myelocytes, 14% promyelocytes, 1% blasts, and 11% lymphocytes. Review of the peripheral blood and bone marrow aspirate revealed an increased number of left-shifted leukocytes with hypolobation and pseudo Pelger-Huët morphologies. A bone marrow aspirate was hypercellular without increased blasts; there was subtle dyserythropoiesis, and dysmegakaryopoiesis primarily consisting of hypolobated megakaryocytes with separate nuclear lobes. The bone marrow biopsy was hypercellular (95%) with a myeloid:erythroid ratio of 5:1. Cytogenetics showed trisomy 8 and no Ph chromosome. The patient was referred for a second opinion. Pathology was confirmed, and next-generation sequencing revealed CSF3R T618I (43% mutant allele frequency) and U2AF1 Q157T (48% mutant allele frequency) mutations. Treatment options were reviewed.
Natural history and prognostic factors
In an Italian cohort of 55 WHO-defined aCML cases,8 the overall median survival was 25 months compared with survivals of 14 to 30 months derived from 3 smaller studies.9-11 A recent US multicenter study applied WHO 2008 criteria to compare aCML (n = 65) and MDS/MPN, U (n = 69) cases and found that the former exhibited a more aggressive clinical course, with respective median overall survivals of 12.4 and 21.8 months.6 In the US and Italian studies,7,8 transformation to acute myeloid leukemia occurred in 37% and 40% of the patients, with a median time to transformation of 11.2 and 18 months, respectively. Increased WBC count (eg, cutoffs of >40 × 109/L or 50 × 109/L), increased percentage of peripheral blood immature precursors, female sex, and older age have been shown to be adverse prognostic factors for overall survival or leukemia-free survival in multivariate analyses.7,8
Molecular and cytogenetic features
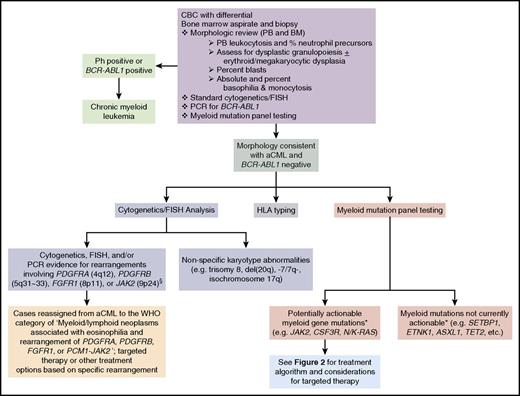
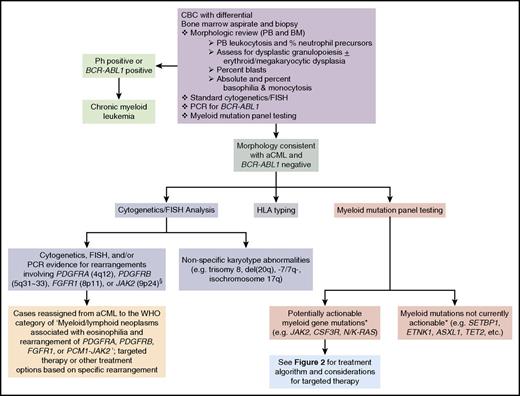
In cases where subtle dysplasia or borderline levels of myeloid immaturity or monocytosis are present, morphologic distinction between aCML, BCR-ABL1-positive CML, CMML, CNL, or MDS/MPN, U can be challenging. In addition to histopathologic analysis of the peripheral blood and bone marrow, modern evaluation of aCML should include next-generation sequencing vis-à-vis myeloid mutation panel testing in addition to standard karyotyping.1-7,12 Diagnosis of aCML first requires testing for the Ph chromosome and/or the BCR-ABL1 fusion gene to exclude CML.1,2 Standard karyotyping, fluorescence in situ hybridization (FISH), and myeloid mutation testing not only complement morphologic analyses, but may also identify opportunities for targeted therapy (Figure 1).
Diagnostic evaluation for aCML and identification of opportunities for targeted therapy. If a morphologic diagnosis of aCML is rendered, cytogenetic, FISH, and myeloid mutation panel testing are critical as they can unmask karyotypic or molecular abnormalities that have potential implications for use of targeted therapy approaches. *, The ability to target certain genes is expected to change over time as new therapeutics are developed. §, Additional JAK2 rearrangements besides the PCM1-JAK2 fusion may present with morphologic features of aCML. BM, bone marrow; PB, peripheral blood; PCR, polymerase chain reaction.
Diagnostic evaluation for aCML and identification of opportunities for targeted therapy. If a morphologic diagnosis of aCML is rendered, cytogenetic, FISH, and myeloid mutation panel testing are critical as they can unmask karyotypic or molecular abnormalities that have potential implications for use of targeted therapy approaches. *, The ability to target certain genes is expected to change over time as new therapeutics are developed. §, Additional JAK2 rearrangements besides the PCM1-JAK2 fusion may present with morphologic features of aCML. BM, bone marrow; PB, peripheral blood; PCR, polymerase chain reaction.
In contrast to BCR-ABL1, which operationally defines CML, no single genetic lesion characterizes aCML. The mutations identified in aCML are commonly found in other myeloid neoplasms.3-7,12-15 The variability that exists in the reported frequency of specific mutations in aCML may partly reflect the stringency to which the WHO definition of aCML was applied in different publications.3 However, some basic observations can be made about the molecular landscape of aCML: higher frequency mutations (eg, >20%) include SETBP1, ASXL1, N/K-RAS, SRSF2, and TET2, and lower frequency mutations (<10%) include CBL, CSF3R, JAK2, and ETNK1.3-7,12-15
Recurrent SETBP1 mutations have been identified in ∼25% to 33% of aCML patients and represent one of the mostly frequently mutated genes in this disease.13-15 Set binding protein (SETBP1) interacts with SET, a negative regulator of the tumor suppressor protein phosphatase 2A (PP2A).16 SETBP1 protects SET from protease cleavage, resulting in an increased amount of SET available to repress PP2A activity.17
Most SETBP1 mutations are located within a 14-amino-acid stretch (codons 858-871), which is also mutated in Schinzel-Giedion syndrome, a rare genetic disease characterized by congenital malformations, mental retardation, and frequent epithelial tumors.18 Normally, phosphorylation of this region leads to binding by E3 ubiquitin ligase subunit b-TrCP1, resulting in ubiquitination and subsequent degradation of SETBP1.13 SETBP1 mutants disrupt this consensus b-TrCP motif, leading to increased SETBP1 and SET expression, which decreases PP2A activity and increases cellular proliferation.13
SETBP1 mutations are associated with a higher leukocyte count, lower hemoglobin and platelet counts, and worse overall survival.13,14 In 1 study, SETPB1 mutations were associated with the presence of −7 and isochromosome i(17)(q10) cytogenetic abnormalities, as well as ASXL1 and CBL mutations, but were mutually exclusive of mutations in the TET2 and JAK2 genes.14 However, SETBP1 is a ubiquitous molecular abnormality among myeloid neoplasms, including CNL and CMML, and may be found in tandem with other mutations, such as with CSF3R in cases of CNL.3,6,14,15,19
Although originally reported at a higher frequency in aCML,20 subsequent reports indicate that the activating CSF3R T618I mutation is present in <10% of cases.1,3-7,12,15 Identification of CSF3R T618I in the context of neutrophilic leukocytosis strongly favors a diagnosis of CNL where it is present in ∼80% of patients.1,3,20,21 Although T618I is the most common activating mutation in CSF3R, an alternative proximal membrane mutation, T640N, has been described in a case of MDS that exhibited transformation to a secondary aCML-like picture.22 Rarely, nonsense and frameshift mutations that result in truncation of the cytoplasmic tail of CSF3R have been found in cases of aCML and are similar to those identified in patients with severe congenital neutropenia who have been administered granulocyte colony-stimulating factor therapy.3,6,20 Evolution of a case of MPN, U to a phenotype of aCML was associated with new subclones of both CSF3R proximal membrane (T618I, 35% mutant allele frequency) and truncation (Q739*, 30% mutant allele frequency) mutants.23
JAK2 V617F is an uncommon mutation (3% to 7%) in aCML and its identification (as well as similar Ph− MPN-associated mutations in CALR and MPL) tends to favor an alternative diagnosis such as PV, ET, or myelofibrosis in the appropriate clinicopathologic context.1,4-7,12,15 More recently, mutations in the ethanolamine kinase 1 (ETNK1) gene were found in 9% of aCML cases24 and were also enriched in patients with CMML and systemic mastocytosis with associated eosinophilia.25 KRAS/NRAS mutations were identified in 7/20 (35%) aCML patients in the aforementioned US multicenter analysis.7 Although SETBP1 and ETNK1 mutations are not yet druggable targets, mutated CSF3R, JAK2, and RAS are important to identify because clinical trial or off-label opportunities for targeted therapy against these lesions may be available (see “Treatment” and “Future prospects” sections; Figures 1 and 2).
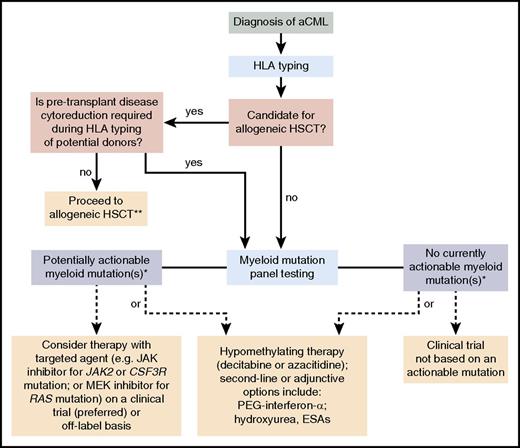
Treatment algorithm for aCML. Please refer to “Treatment” for a discussion of this treatment scheme for aCML. This algorithm is based on several decision nodes, including the following: (1) potential candidacy for allogeneic hematopoietic stem cell transplantation (HSCT); (2) the results of myeloid mutation panel testing; (3) eligibility for enrollment in clinical trials; and (4) opportunities to adopt strategies used for MDS or MPN (eg, hypomethylating agents or second-line/adjunctive therapies). ESAs, erythropoiesis-stimulating agents. *, The ability to target certain genes is expected to change over time as new therapeutics are developed. **, Myeloid mutation panel testing may also be performed prior to patients proceeding directly to allogeneic HSCT who do not require pretransplant disease cytoreduction.
Treatment algorithm for aCML. Please refer to “Treatment” for a discussion of this treatment scheme for aCML. This algorithm is based on several decision nodes, including the following: (1) potential candidacy for allogeneic hematopoietic stem cell transplantation (HSCT); (2) the results of myeloid mutation panel testing; (3) eligibility for enrollment in clinical trials; and (4) opportunities to adopt strategies used for MDS or MPN (eg, hypomethylating agents or second-line/adjunctive therapies). ESAs, erythropoiesis-stimulating agents. *, The ability to target certain genes is expected to change over time as new therapeutics are developed. **, Myeloid mutation panel testing may also be performed prior to patients proceeding directly to allogeneic HSCT who do not require pretransplant disease cytoreduction.
Nonspecific karyotypic abnormalities have been reported in a moderate proportion of aCML patients. These include single or double abnormalities, or complex cytogenetics, including trisomy 8, del(20q), −7/7q-, or isochromosome 17q [i17[q)].2,7,8,10,11 Notably, the literature includes patients with rearrangement of PDGFRA, PDGFRB, FGFR1, or PCM1-JAK2 who have been given the diagnosis of aCML.1,2,26-30 Although in some of these cases the term “aCML” may have been loosely applied to indicate a CML-like disease in the absence of BCR-ABL1, other cases may truly fulfill morphologic criteria for aCML. However, according to the WHO classification, the presence of any of these genetic rearrangements reassigns such cases to the major category of “Myeloid/lymphoid neoplasms associated with eosinophilia and rearrangement of PDGFRA, PDGFRB, FGFR1, or PCM1-JAK2.”1,2 Although not formally included in this category, cases with rearranged FLT3 may rarely morphologically mimic aCML.31 Recognizing the relevant break points for reciprocal translocations that infer involvement of PDGFRA (4q12; excluding the most common FIP1L1-PDGFRA rearrangement, which is not visible by standard karyotyping), PDGFRB (5q31∼33), FGFR1 (8p11), and JAK2 (9p24) is also critical for recognizing instances where use of tyrosine kinase inhibitors such as imatinib should be considered (eg, rearranged PDGFRA/B with confirmation by FISH or sequencing) or where poor disease prognosis disease mandates high-intensity approaches such as induction chemotherapy and/or allogeneic HSCT (eg, for patients with rearranged FGFR1).
Treatment
No standard of care exists for the treatment of aCML. In addition, no consensus recommendations or risk-based treatment algorithms exist to help guide a watch-and-wait approach vs initiation of therapy. However, progressive leukocytosis, anemia and/or thrombocytopenia, or emergence of symptomatic splenomegaly or disease-related constitutional symptoms should prompt treatment.
Given its unfavorable prognosis, my treatment algorithm (Figure 2) is first to consider HSCT for eligible patients with a suitable donor without initially relying on the results of myeloid mutation testing. Although the best timing of transplantation (eg, earlier in the course of disease or at the time of disease progression) remains an unresolved question, the otherwise poor outcomes of aCML patients should encourage evaluation of the feasibility of this treatment modality after the diagnosis is made. If a donor is not immediately available and/or disease cytoreduction is recommended, I consider the results of myeloid mutation testing to evaluate clinical trial options (preferred) or off-label opportunities with targeted therapy. Current examples include ruxolitinib for CSF3R- or JAK2-mutated patients, or MEK inhibition in RAS-mutated patients (see “Future prospects”). Regardless of the results of mutation testing, hypomethylating therapy may be considered in such individuals because the prognostic relevance of these mutations to treatment response is unknown. I similarly use the results of myeloid mutation panel testing for patients who are not transplant candidates to evaluate opportunities for trials of targeted therapy. If no such option exists, my approach is to consider hypomethylating therapy or clinical trials of novel therapies not based on an actionable mutation. Additionally, I co-opt treatment strategies used for either MDS or MPN and apply them on a case-by-case basis to address a patient’s major clinical issues (eg, leukocytosis, anemia, constitutional symptoms, splenomegaly, and potential for progression to acute myeloid leukemia). These second-line or adjunctive options may include PEG-IFN-α, hydroxyurea, and/or ESAs.
HSCT
A limited number of HSCT procedures for aCML have been published. Most are included in series of patients with heterogeneous MDS/MPN where long-term disease free–survival of 40% to 50% has been recorded.3,32,33 Koldehoff and colleagues’ retrospective analysis of 9 individuals with aCML represents the largest transplantation series focused solely on this disease.34 In this series, allogeneic donor types consisted of an HLA-identical sibling (N = 4), or unrelated matched donor (n = 4); 1 patient underwent syngeneic transplantation from a twin sibling. Conditioning regimens included cyclophosphamide with either total body irradiation (n = 5), busulfan (n = 2), or bulsulfan and alemtuzumab (n = 1); and in 1 older patient, a reduced intensity conditioning (RIC) regimen consisting of busulfan, fludarabine, and antithymocyte globulin was used. All patients achieved a complete remission; the patient who received bone marrow from his brother relapsed 19 months after transplant but was successfully retransplanted with peripheral blood stem cells from this donor. Chronic graft-versus-host disease (GVHD) was observed in all allografted patients, and grade 2 to 4 acute GVHD occurred in 5 of 8 patients (63%). The patient who received alemtuzumab developed cerebral toxoplasmosis and died of sepsis 273 days posttransplant. A follow-up report by this group indicated that 21 patients with aCML had been transplanted, with 17 of 21 patients alive at 5 years after transplantation with a median survival of 47 months.35 These analyses compare more favorably to another study of allogeneic transplantation that included 7 patients with Ph chromosome–negative/BCR-AB1-negative CML.36 One patient suffered relapse at 9 months, and 5 of the patients had died by 3 to 26 months of follow-up.
A more recent retrospective study evaluating allogeneic HSCT in 10 MDS/MPN patients included 2 patients with aCML who received busulfan/cyclophosphamide conditioning with bone marrow allografts from matched sibling donors.33 Both patients remained alive with no evidence of disease after 96 to 99 months of follow-up. Notably, relapse was observed in the 5 of 10 MDS/MPN patients who received RIC compared with none of the patients who received myeloablative conditioning. Because many patients with aCML or other MDS/MPN are elderly and may only be eligible for RIC, novel strategies are needed to reduce relapse in such individuals.
Molecular profiling to identify poor-risk mutations such as SETBP1 and ASXL1 may prompt earlier consideration of HSCT for eligible aCML patients. It is currently unknown whether HSCT can modify the adverse prognosis related to these mutations in the context of aCML. However, among 36 MDS/MPN patients with DNA available for serial molecular analysis, survival after HSCT was not influenced by ASXL1, CBL, NRAS, or TET2 mutations (SETBP1 was not assessed).32 Detection of these pretransplant molecular markers, as well as CSF3R T618I may be useful for serial monitoring of minimal residual disease after HSCT, as their detection has been associated with overt relapse.37
Hypomethylating agents
The use of hypomethylating agents in aCML is a rational application of their established activity in MDS and CMML. Among CMML patients treated in phase 2 studies of hypomethylating agents, overall response rates range from 25% to 70% (average 30% to 40%), with overall survival ranging from 12 to 37 months (reviewed in Patnaik and Tefferi38 ). It is challenging to extrapolate from the limited data on proliferative CMML patients to broadly inform how patients with aCML may respond. However, in 1 analysis, a WBC count >13 × 109/L and bone marrow blasts >10% were adverse prognostic factors for response to azacitidine.39
I consider the use of azacitidine or decitabine in the following 2 scenarios: (1) as a bridging therapy for those who are eligible for HSCT; and (2) as stand-alone treatment of patients without a HSCT or clinical trial option. However, the experience with hypomethylating agents in aCML is limited and cannot be considered a standard of care. Decitabine (20 mg/m2 daily IV × 5 days) produced a complete hematologic remission (CHR) in 7 of the 8 patients described in 4 separate reports.40-43 Patients received a total number of 1 to 6 cycles; 4 patients achieved a CHR after 1 course of decitabine, and 3 patients achieved a CHR after 4 cycles. Response duration and length of follow-up were variably described in these publications; in 1 report, 2 patients were alive at 9 to 15 months after initiation of therapy,41 and 1 patient who maintained a response for 20 months died at 26 months of follow-up. Two patients treated with decitabine were successfully bridged to transplant, with 1 dying of GVHD, BK viremia, and multiorgan failure on day +49.42 The small number of patients treated thus far precludes a determination of which clinical, laboratory, or molecular markers predict for response.
Ruxolitinib
Although uncommon, the identification of CSF3R T618I or JAK2 V617F in cases of aCML provides an opportunity to consider JAK inhibitor therapy because both of these mutations result in JAK-STAT pathway activation. Preclinical studies indicate that ruxolitinib potently inhibits CSF3R T618I–driven malignant cell growth and can reduce leukocytosis and spleen size in a lethal myeloproliferative disease in mice driven by the mutation.44 However, such data do not supplant my recommendation to first consider HSCT for all eligible aCML patients, including individuals with either of these druggable mutations. JAK inhibition may useful to consider as a bridge to allogeneic HSCT; in the context of myelofibrosis, clinical improvement (vs no clinical improvement) with JAK inhibitors (defined by International Working Group–Myeloproliferative Neoplasms Research and Treatment response criteria) was associated with improved overall survival after transplant in a multivariate analysis45 and may relate to improvement of performance status and reduction of splenomegaly.
Ruxolitinib is the only JAK inhibitor currently approved by the US Food and Drug Administration (for patients with intermediate or high-risk myelofibrosis, or for patients with PV demonstrating intolerance or resistance to hydroxyurea). I recommend that patients be treated with this agent in the context of a clinical trial. Currently, a multicenter study (#NCT02092324) is evaluating the safety and efficacy of ruxolitinib in patients with CNL and aCML, regardless of mutation status. However, if clinical trial enrollment is not feasible, I would consider off-label use of ruxolitinib in CSF3R T618I–mutated or JAK2 V617F–mutated patients.
The potential benefit of ruxolitinib in CSF3R T618I–mutated disease was first demonstrated in a patient with CNL with CSF3R T618I who achieved a marked reduction in neutrophilic leukocytosis and improvement of anemia and thrombocytopenia.20 Subsequently, a patient with hydroxyurea-refractory aCML dosed with ruxolitinib 10 to 20 mg twice daily resulted in similar hematologic improvements.46 Clinical benefits experienced by the patient included reduction of peripheral blood myeloid immaturity, marrow granulocytic hyperplasia, and dysplastic megakaryocytes. In addition, ruxolitinib decreased in spleen volume by 75% after 3 months of therapy, reverted weight loss, and improved symptom scores. However, no change in CSF3R T618I mutant allele frequency was observed.
In the pediatric setting, ruxolitinib (50 mg/m2) has been used in an 11-year-old girl with aCML.47 Ruxolitinib decreased the leukocyte count from 101 × 109 to 7.9 × 109/L after 1 week, ultimately permitting the patient to be bridged to a successful allogeneic HSCT. Although not well studied, the presence of additional mutations besides CSF3R T618I, such as SETBP1, may reduce responsiveness to JAK inhibitor therapy in aCML.48 Given its similar oncogenicity to CSF3R T618I in cellular transformation assays and in an in vivo murine transplantation model, aCML patients with the rarely described CSF3R T640N mutation would also be predicted to respond to JAK inhibition. As previously noted, patients with morphologic presentations consistent with aCML may exhibit rearrangements of JAK2, most notably PCM1-JAK2, which are sensitive to JAK inhibition, but with variable response duration.49-51 In contrast to CSF3R membrane proximal mutations, CSF3R truncation mutants preferentially activate the downstream SRC family kinases and TNK2. Although in vitro assays showed that dasatinib could inhibit colony formation from bone marrow cells transduced with truncation mutant-CSF3R-containing retroviruses,20 the efficacy of dasatinib in patients with these mutations has not yet been reported.
Other medical therapies
Complete and partial hematologic remissions have been reported with hydroxyurea in Ph chromosome/BCR-ABL1-negative CML patients, but the remissions are usually short lived.9,10,52-54 Similar to its role in MPNs, I use hydroxyurea as a supportive care measure either alone or as an adjunct to other therapies to control leukocytosis or progressive, symptomatic splenomegaly. Older studies evaluating standard IFN-α noted partial or complete hematologic remitting activity with variable durability of response.9,10,52-54 In a phase 2 study of PEG-IFN-a-2b (starting dose of 3 μg/kg per week), 2 of 5 BCR-ABL1-negative CML patients achieved complete remission after 3 months of therapy.55 Median duration of therapy was 36 and 38 months at which time both patients were discontinued because of toxicity. Given the more favorable toxicity profile of PEG-IFNs, these extended formulations merit further investigation in aCML. Because treatment of anemia remains an unmet need, it may also be fruitful to explore whether factors predicting response to ESAs in MDS56 also have potential applicability to aCML.
Splenectomy and splenic irradiation have limited roles in the management of aCML.9,10,52 In rare circumstances, either therapeutic modality may be useful for disease palliation when other options have failed. However, the use of either modality must be weighed against morbid complications such as bleeding, thrombosis, infection, and potential for acceleration of leukocytosis and hepatomegaly.
Return to the cases
Based on the limited data available for aCML therapy, and the diagnostic and treatment algorithms I have outlined, we now return to the disposition of the 2 cases.
Case 1
We obtained a myeloid mutation panel on patient LJ which revealed 3 pathogenic variants: SETBP1 G870S (mutant allele frequency 45%), SRSF2 P95H (mutant allele frequency 50%), and ASXL1 P808fs*10 (1 bp deletion with frame shift; mutant allele frequency 45%). Although the patient’s WBC count was only mildly elevated and the hemoglobin and platelets were well preserved, we discussed our concern about aCML-associated survival and her molecular profile, specifically the unfavorable prognosis associated with SETBP1 and the generally poor-risk related to ASXL1 and SRSF2 in the context of other myeloid neoplasms. The patient underwent consultation for a reduced-intensity conditioning HSCT and for HLA typing. Because her 2 siblings were not matches, an unrelated donor search was initiated. Over the next 2 months, her WBC count increased to 48 × 109/L, and progressive cytopenias developed (hemoglobin, 9.5 g/dL; platelets, 85 × 109/L). A repeat bone marrow revealed increased blasts (8%) without clonal cytogenetic evolution. We recommended decitabine therapy (20 mg/m2 IV × 5 days on 28-day cycles). After 3 cycles, she achieved a CHR, and a repeat bone marrow showed 3% blasts with persistent trilineage dysplasia. A 10/10 unrelated donor was identified and the patient proceeded to a RIC HSCT. She remains in a hematologic and molecular remission 15 months after transplant with mild-moderate chronic GVHD.
Case 2
This patient’s aCML was likely masked by treatment of his hepatitis C with PEG-IFN-α-2a. With stoppage of treatment, laboratory features of aCML emerged. Because of CK’s age and performance status, a mutual decision was made by the patient and physician to seek alternative treatment besides HSCT. Because he carried the CSF3R T618I mutation (albeit uncommon in aCML), he decided to pursue a clinical trial with ruxolitinib. In order to be eligible, he first underwent treatment with ledipasvir/sofobuvir which eradicated his hepatitis C. Before trial initiation, his complete blood count revealed a WBC count of 32.7 × 109/L, hemoglobin of 9.5 g/dL, and platelet count of 57 × 109/L, and the spleen had increased to 7 cm below the left costal margin. No significant changes in the bone marrow were observed except a borderline increase in blasts (5%). Treatment with 4 months of ruxolitinib in the range of 5 to 10 mg twice daily eliminated splenomegaly and markedly improved his symptom burden. In addition, the WBC count decreased to 11.9 × 109/L, and the platelet count improved to 130 × 109/L; however, the patient became red blood cell transfusion dependent. After 2 additional months of therapy, the WBC and platelet count began to worsen again. A repeat marrow demonstrated a further increase in blasts to 10%. The patient has recently been switched to decitabine therapy.
Future Prospects
In this era of precision medicine, it is incumbent on physicians evaluating aCML patients to employ myeloid mutation panels to uncover potentially druggable targets. A recent example comes from investigators from Oregon Health and Sciences University who identified an NRAS G12D mutation at 47% mutant allele frequency in an aCML patient.57 The mitogen-activated protein kinase kinase 1 (MEK1)/MEK2 inhibitor tramenitinib, approved for malignant melanoma, also exhibits activity in RAS-driven leukemias in vitro and in vivo.58,59 Treatment with trametinib 2 mg daily produced a durable near-complete hematologic response, with the WBC count decreasing from 256 × 109/L to the 10 × 109 to 15 × 109/L range and the platelet count improving from 66 × 109/L to 168 × 109/L.57 Pharmacologic reactivation of the tumor suppressor PP2A, which is functionally suppressed because of SETPB1 mutations, is a promising therapeutic approach to pursue with drugs such as fingolimod (FTY720).60,61 Spliceosome modulators are a novel class of therapeutics entering clinical trials for myeloid neoplasms and may have a role in aCML patients with mutations in SRSF2 or other genes that comprise the spliceosome machinery.62 Combination strategies employing therapies targeting disease-associated mutations in conjunction with either (1) hypomethylating agents or (2) as an adjunct to HSCT (either as a bridge to transplant or in the posttransplant setting to reduce relapse) should be evaluated. Lastly, I encourage use of the new international MDS/MPN response criteria so that treatment responses between regimens can be more accurately compared.63
Acknowledgments
The author thanks Kim-Hien Dao, Julia Maxson, and Jeffrey Tyner for their research collaboration on the molecular pathogenesis of CNL and aCML, as well as Tracy George and Dan Arber for their input regarding the WHO classification of MDS/MPN.
J.G. is supported by the Charles and Ann Johnson Foundation.
Authorship
Contribution: J.G. alone wrote this manuscript.
Conflict-of-interest disclosure: J.G. receives research funding as a subinvestigator of an Incyte-sponsored trial of ruxolitinib in patients with CML and aCML. He also receives honoraria and has participated on advisory boards for Incyte Inc.
Correspondence: Jason Gotlib, Stanford Cancer Institute, 875 Blake Wilbur Dr, Room 2324, Stanford, CA 94305-5821; e-mail: jason.gotlib@stanford.edu.