Key Points
BCR-ABL1–positive cells outside the B-lineage compartment are found in 40% of adult patients with BCR-ABL1–positive BCP-ALL.
Selection of preexisting CD19– subclones is a potential source of tumor escape after CD19-targeted therapies in adult Philadelphia chromosome–positive ALL.
Abstract
The bispecific T-cell engager blinatumomab targeting CD19 can induce complete remission in relapsed or refractory B-cell precursor acute lymphoblastic leukemia (BCP-ALL). However, some patients ultimately relapse with loss of CD19 antigen on leukemic cells, which has been established as a novel mechanism to escape CD19-specific immunotherapies. Here, we provide evidence that CD19-negative (CD19–) relapse after CD19-directed therapy in BCP-ALL may be a result of the selection of preexisting CD19– malignant progenitor cells. We present 2 BCR-ABL1 fusion–positive BCP-ALL patients with CD19– myeloid lineage relapse after blinatumomab therapy and show BCR-ABL1 positivity in their hematopoietic stem cell (HSC)/progenitor/myeloid compartments at initial diagnosis by fluorescence in situ hybridization after cell sorting. By using the same approach with 25 additional diagnostic samples from patients with BCR-ABL1–positive BCP-ALL, we identified HSC involvement in 40% of the patients. Patients (6 of 8) with major BCR-ABL1 transcript encoding P210BCR-ABL1 mainly showed HSC involvement, whereas in most of the patients (9 of 12) with minor BCR-ABL1 transcript encoding P190BCR-ABL1, only the CD19+ leukemia compartments were BCR-ABL1 positive (P = .02). Our data are of clinical importance, because they indicate that both CD19+ cells and CD19– precursors should be targeted to avoid CD19– relapses in patients with BCR-ABL1–positive ALL.
Introduction
B-cell–directed therapies have shown promising results in the treatment of chemotherapy-resistant or relapsed B-cell precursor acute lymphoblastic leukemia (BCP-ALL). Therapy with blinatumomab, a bispecific T-cell engager antibody that links CD3+ T cells to CD19+ B cells and thereby triggers serial lysis of B cells, results in a complete response rate of up to 69% in relapsed or refractory BCP-ALL.1-4 Similarly, therapy with CD19-specific chimeric antigen receptor-modified T cells (CART-19) induces complete response in up to 90% of patients with BCP-ALL.5,6 However, a considerable proportion of patients relapse after an initial molecular response, whereas others remain in a lasting remission after CD19-directed treatment. CD19– relapses have been described for both therapy settings.1,5,7-9 Epitope loss resulting from mutations, alternative splicing, or disrupted CD19 membrane trafficking that pretends CD19 negativity may contribute to resistance against CART-19 therapy.9,10 Furthermore, a few patients with CD19– relapse have been described which appear as acute myeloid leukemia, indicating a previous lineage switch and a real CD19 negativity.8,11 The mechanism behind this phenomenon, such as the cell of origin or clonal evolution in those patients, has not been described until now. By using fluorescence in situ hybridization (FISH) after fluorescence-activated cell sorting (FACS) in 2 patients with BCR-ABL1–positive BCP-ALL with relapse after blinatumomab and in 25 additional diagnostic samples from patients with BCR-ABL1–positive BCP-ALL, we are able to provide evidence that CD19– myeloid lineage relapses in adult patients with BCR-ABL1–positive BCP-ALL occur in association with hematopoietic stem cell (HSC) involvement.
Study design
Cryoconserved bone marrow and peripheral blood samples from adult patients with BCR-ABL1–positive BCP-ALL treated within 4 different trials were subjected to FACS, placed onto slides, and analyzed by FISH for BCR-ABL1 fusion (LSI BCR/ABL Dual Color, Dual Fusion Translocation probe; Abbott Laboratories, Abbott Park, IL) (cutoff was set to 3%). The 4 trials were GMALL Elderly 1/2003 (NCT00198978; German Multicenter Trial for Treatment of Elderly Patients With Newly Diagnosed Acute Lymphoblastic Leukemia), 07/2003 (NCT00198991; German Multicenter Trial for Treatment of Newly Diagnosed Acute Lymphoblastic Leukemia in Adults), MT103-206 (NCT01209286; Study of the bispecific T-cell engager [BiTE] Blinatumomab [MT103] in Adult Patients With Relapsed/Refractory B-Precursor Acute Lymphoblastic Leukemia [ALL]), and Alcantara (NCT02000427; A Phase 2 Single Arm, Multicenter Trial to Evaluate the Efficacy of the BiTE Antibody Blinatumomab in Adult Subjects With Relapsed/Refractory Philadelphia Positive B-precursor Acute Lymphoblastic Leukemia). Patient characteristics are provided in supplemental Table 1, available on the Blood Web site. In patient 21, a centromere 7 probe (CEP7, Abbott Laboratories) (cutoff was defined as 3%) was added to the BCR-ABL1 fusion probe. The following populations were investigated on the basis of the approach described by Castor et al12 : CD34+38–19–3– (HSCs and multipotential progenitor [MPP] cells), CD34+38+19–3– (myeloid and lymphoid progenitors), CD34+19+20–3– (leukemia cells without CD20 coexpression; leukemia associated immunophenotype [LAIP] 20–), CD34+19+20+3– (leukemia cells with CD20 coexpression; LAIP 20+), CD34–19+20+ (mature B cells), CD34–19–20–3+ (mature T cells), CD34+19–13/33+10–16– (early myeloid compartment), CD34–19–13/33+10–16– (late myeloid compartment), and CD34–19–13/33+10–16+ (mature myeloid compartment) (supplemental Figure 1; supplemental Tables 2-4; supplemental Methods). Cell sorting, FISH, array comparative genomic hybridization, molecular analysis of immunoglobulin (IG) and T-cell receptor (TR) gene rearrangements, and BCR-ABL1 real-time quantitative polymerase chain reaction were performed as described in supplemental Methods. The clinical study was approved by the local ethics committee (D448/14). Informed consent was obtained in accordance with the Declaration of Helsinki.
Results and discussion
Patient 21 presented with a relapse of BCR-ABL1–positive BCP-ALL after standard induction and consolidation treatment. She received blinatumomab monotherapy and achieved rapid complete molecular response. However, within the third application cycle of blinatumomab, BCR-ABL1–positive blasts exhibiting a CD19– myeloid phenotype reoccurred (Figure 1A). Molecular analysis did not show clonal IG/TR gene rearrangements in the relapse bulk, which suggested that leukemic relapse derived from cells of a CD19– progenitor compartment (Figure 1B). Array comparative genomic hybridization revealed a monosomy 7 at initial diagnosis but not at relapse (supplemental Figure 2). To elucidate the clonal evolution of malignancy, at initial diagnosis, bone marrow cells were subjected to FACS for distinct cellular compartments and were subsequently analyzed by FISH with a 3-color BCR-ABL1/CEP7 probe (Figure 1C). Indeed, BCR-ABL1 fusion/monosomy 7–positive cells were found in the CD19+ leukemia compartments (CD34+19+20–CD3– and CD34+19+20+CD3–), but they were also found within the HSC and progenitor compartments (CD34+38–19–3– and CD34+38+19–3–) and within the myeloid compartment (CD34+19–13/33+10–16–, CD34–19–13/33+10–16–, and CD34–19–13/33+10–16+). BCR-ABL1 fusion–positive cells without monosomy 7 were also present at initial diagnosis within the myeloid but not within the CD19+ leukemia compartment. Within the HSC and progenitor cell compartments they remained at 4% and 3%, respectively, which was within the FISH and sorting purity cutoff range. The same analysis was performed in a second patient (patient 29) with BCR-ABL1-positive BCP-ALL who suffered a CD19– myeloid relapse after blinatumomab therapy. The progenitor and subsequent myeloid compartments in this patient also showed BCR-ABL1 positivity at initial diagnosis.
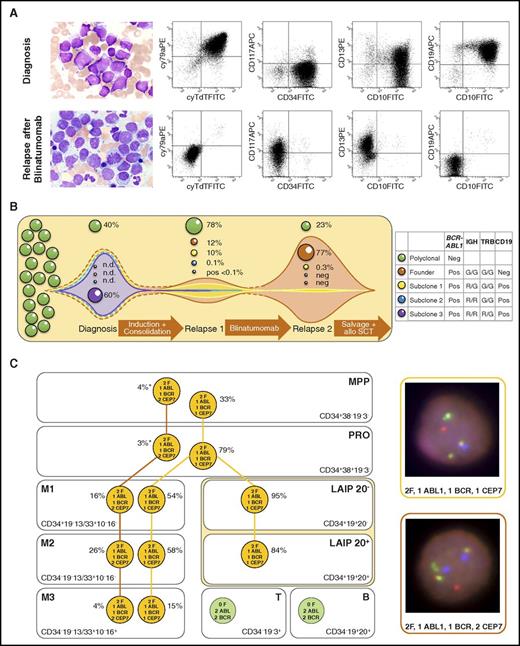
Leukemic involvement and evolution of BCR-ABL1–positive blasts in patient 21. (A) Blast morphology and flow cytometric dot plots of blasts before blinatumomab (first row) and blasts after blinatumomab (second row). Cytomorphology images were acquired using a 63×/1.40 numeric aperture oil objective in a Zeiss Axioplan 2 microscope (Zeiss, Jena, Germany) after Pappenheim's staining (panoptic staining). Blasts at initial diagnosis were positive for CD19, CD10, cyCD79a, CD34, TdT, cyCD22*, HLA-DR*, and cyIgM*; showed aberrant expression of CD13; and were negative for CD117, CD33*, and MPO*. The expression profile did not fulfill World Health Organization criteria for classification as mixed-phenotype leukemia. At relapse after blinatumomab therapy, blasts were negative for CD19, CD10, cyCD79a, CD34, TdT, HLA-DR*, and MPO* and expressed myeloid antigens CD117, CD13, and CD33* (* indicates respective antigen expression not shown in the dot blots). (B) Hypothetical model of clonal evolution and selection of different subclones based on BCR-ABL1 and immunoglobulin heavy chain (IGH) and T-cell receptor β (TRB) gene rearrangement patterns (figure not to scale). Patients with leukemia were screened at initial diagnosis for clonal IG and TR gene rearrangements. Two clonal IGH gene rearrangements (VH3-23-DH2-2-JH6 and VH6-1-DH3-22-JH4) and 1 clonal cross-lineage TRB gene rearrangement (DB2-JB2.7) were detected, and clone-specific real-time quantitative polymerase chain reaction (RT-qPCR) assays were established on the basis of sequence information. RT-qPCR and BCR-ABL1 FISH showed dominance of the IGH R/R, TRB R/G, and BCR-ABL1–rearranged clone (R, rearranged; G, germ line). At first relapse, the IGH R/G TRB G/G clone was dominant, but the second IGH rearrangement was detected at a level of only 0.1% and TRB only at a level below the quantitative range of 0.1%. At second relapse, the leukemic bulk did not show an IG/TR gene rearrangement, but only the BCR-ABL1 translocation RT-qPCR revealed a subclonal IGH gene rearrangement (0.3%). A clonal evolution of the leukemic bulk with occurrence of a new dominant IGH/TRB gene rearrangement was excluded by IGH/TRB multiplex PCR, which has a sensitivity of about 1% to 5%. (C) Subclonal architecture of BCR-ABL1 fusion and monosomy 7 in immunophenotypic compartments of patient 21 at initial diagnosis analyzed by FISH after FACS. Left: FISH results of each compartment. Orange circle, aberrant signal constellation; green circle, normal signal constellation; asterisk (*) indicates being within the range of the FISH and sorting purity cutoff. Right: Representative interphase nuclei showing the 2 different aberrant signal constellations in a false color display using MetaSystems software. FISH images were acquired using a 63×/1.40 numeric aperture oil objective in a Zeiss Axioskop 2 fluorescence microscope (Axioskop 2). The meaning of signals is as follows: isolated red, ABL1; isolated green, BCR; red-green fusion signal, BCR-ABL1 fusion; blue, centromere 7. APC, allophycocyanin; B, mature B cells; CEP7, centromere 7 signal; F, BCR-ABL1 fusion signal; FITC, fluorescein isothiocyanate; leukemia-associated immunophenotype 20− (LAIP 20−), leukemic bulk without CD20 coexpression; LAIP 20+, leukemic bulk with CD20 coexpression; M1, early myeloid compartment; M2, late myeloid compartment; M3, mature myeloid compartment; MPP, multipotent progenitor cells; n.d., not determined; neg., negative (not detected); PE, phycoerythrin; pos, positive; PRO, myeloid and lymphoid progenitors; SCT, stem cell transplantation; T, mature T cells.
Leukemic involvement and evolution of BCR-ABL1–positive blasts in patient 21. (A) Blast morphology and flow cytometric dot plots of blasts before blinatumomab (first row) and blasts after blinatumomab (second row). Cytomorphology images were acquired using a 63×/1.40 numeric aperture oil objective in a Zeiss Axioplan 2 microscope (Zeiss, Jena, Germany) after Pappenheim's staining (panoptic staining). Blasts at initial diagnosis were positive for CD19, CD10, cyCD79a, CD34, TdT, cyCD22*, HLA-DR*, and cyIgM*; showed aberrant expression of CD13; and were negative for CD117, CD33*, and MPO*. The expression profile did not fulfill World Health Organization criteria for classification as mixed-phenotype leukemia. At relapse after blinatumomab therapy, blasts were negative for CD19, CD10, cyCD79a, CD34, TdT, HLA-DR*, and MPO* and expressed myeloid antigens CD117, CD13, and CD33* (* indicates respective antigen expression not shown in the dot blots). (B) Hypothetical model of clonal evolution and selection of different subclones based on BCR-ABL1 and immunoglobulin heavy chain (IGH) and T-cell receptor β (TRB) gene rearrangement patterns (figure not to scale). Patients with leukemia were screened at initial diagnosis for clonal IG and TR gene rearrangements. Two clonal IGH gene rearrangements (VH3-23-DH2-2-JH6 and VH6-1-DH3-22-JH4) and 1 clonal cross-lineage TRB gene rearrangement (DB2-JB2.7) were detected, and clone-specific real-time quantitative polymerase chain reaction (RT-qPCR) assays were established on the basis of sequence information. RT-qPCR and BCR-ABL1 FISH showed dominance of the IGH R/R, TRB R/G, and BCR-ABL1–rearranged clone (R, rearranged; G, germ line). At first relapse, the IGH R/G TRB G/G clone was dominant, but the second IGH rearrangement was detected at a level of only 0.1% and TRB only at a level below the quantitative range of 0.1%. At second relapse, the leukemic bulk did not show an IG/TR gene rearrangement, but only the BCR-ABL1 translocation RT-qPCR revealed a subclonal IGH gene rearrangement (0.3%). A clonal evolution of the leukemic bulk with occurrence of a new dominant IGH/TRB gene rearrangement was excluded by IGH/TRB multiplex PCR, which has a sensitivity of about 1% to 5%. (C) Subclonal architecture of BCR-ABL1 fusion and monosomy 7 in immunophenotypic compartments of patient 21 at initial diagnosis analyzed by FISH after FACS. Left: FISH results of each compartment. Orange circle, aberrant signal constellation; green circle, normal signal constellation; asterisk (*) indicates being within the range of the FISH and sorting purity cutoff. Right: Representative interphase nuclei showing the 2 different aberrant signal constellations in a false color display using MetaSystems software. FISH images were acquired using a 63×/1.40 numeric aperture oil objective in a Zeiss Axioskop 2 fluorescence microscope (Axioskop 2). The meaning of signals is as follows: isolated red, ABL1; isolated green, BCR; red-green fusion signal, BCR-ABL1 fusion; blue, centromere 7. APC, allophycocyanin; B, mature B cells; CEP7, centromere 7 signal; F, BCR-ABL1 fusion signal; FITC, fluorescein isothiocyanate; leukemia-associated immunophenotype 20− (LAIP 20−), leukemic bulk without CD20 coexpression; LAIP 20+, leukemic bulk with CD20 coexpression; M1, early myeloid compartment; M2, late myeloid compartment; M3, mature myeloid compartment; MPP, multipotent progenitor cells; n.d., not determined; neg., negative (not detected); PE, phycoerythrin; pos, positive; PRO, myeloid and lymphoid progenitors; SCT, stem cell transplantation; T, mature T cells.
To investigate the clonal architecture of BCR-ABL1 ALL systematically, pretherapeutic samples from an additional 25 patients with BCR-ABL1-positive BCP-ALL were analyzed accordingly (Figure 2). HSC involvement was revealed in 10 (40%) of these patients. Nine of them showed the same pattern of BCR-ABL1 positivity (HSC/progenitor, myeloid, and leukemia involvement) as patients 21 and 29, which we now termed “MPP pattern.” In 13 (52%) of 25 patients, a second predominant pattern, referred to as “B-lineage-pattern,” was identified in which BCR-ABL1 positivity was restricted to the B-lineage-determined CD34+19+ cells. In 23 of 25 evaluable patients, independent of the pattern of BCR-ABL1 positivity, the mature B-cell compartment was BCR-ABL1 negative, which indicated a lymphatic maturation stop of BCR-ABL1-positive cells. Patients with major BCR-ABL1 transcript mainly showed the MPP pattern (6 of 8) and much less frequently showed the B-lineage-pattern (1 of 8). In contrast, patients with minor BCR-ABL1 transcript predominantly showed the B-lineage pattern (9 of 12) and much less frequently showed the MPP pattern (3 of 12) (P = .02; Fisher’s exact test).
Analysis of relevant immunophenotypic compartments in 27 adult patients with BCR-ABL1–positive BCP-ALL using FISH after FACS. (A) BCR-ABL1 positivity of immunophenotypic compartments in the 2 predominant patterns of BCR-ABL1 occurrence. The green and orange color content of the boxes represents the ratios of BCR-ABL1 fusion–positive and –negative signal constellations observed in the 27 patients. For details of sorting strategies, see supplemental Data. (B) Detailed results of the analysis for each patient and compartment. Green, BCR-ABL1 fusion negative; orange, BCR-ABL1 positive; gray, not analyzable. Overall, 11 of 27 patients showed an MPP pattern, 13 of 27 showed a B-lineage pattern, and 3 of 27 had a pattern between the two (indeterminable [i.d.]). M, major BCR-ABL1 transcript; m, minor BCR-ABL1 transcript; mM, minor and major BCR-ABL1 transcripts identified; n.a., not analyzable.
Analysis of relevant immunophenotypic compartments in 27 adult patients with BCR-ABL1–positive BCP-ALL using FISH after FACS. (A) BCR-ABL1 positivity of immunophenotypic compartments in the 2 predominant patterns of BCR-ABL1 occurrence. The green and orange color content of the boxes represents the ratios of BCR-ABL1 fusion–positive and –negative signal constellations observed in the 27 patients. For details of sorting strategies, see supplemental Data. (B) Detailed results of the analysis for each patient and compartment. Green, BCR-ABL1 fusion negative; orange, BCR-ABL1 positive; gray, not analyzable. Overall, 11 of 27 patients showed an MPP pattern, 13 of 27 showed a B-lineage pattern, and 3 of 27 had a pattern between the two (indeterminable [i.d.]). M, major BCR-ABL1 transcript; m, minor BCR-ABL1 transcript; mM, minor and major BCR-ABL1 transcripts identified; n.a., not analyzable.
Both patients with BCR-ABL1-positive BCP-ALL who developed a CD19– myeloid lineage relapse after blinatumomab therapy showed BCR-ABL1-positive cells in the HSC/progenitor and myeloid compartments at initial diagnosis. These results as well as the fact that the IG genes of the CD19– relapses showed germline configuration and not the clonal rearrangements present at initial diagnosis suggest that the CD19– relapses in these patients evolved from CD19–BCR-ABL1-positive progenitor cells. It also indicates that the CD19– myeloid lineage relapses are not a consequence of dedifferentiation or reprogramming. Jacoby et al13 did not identify myeloid leukemic clones prior to therapy when they induced CD19– myeloid lineage relapses in a murine BCP-ALL model by long-term CART-19 exposure. However, this does not disprove the presence of uncommitted leukemic progenitors at low frequencies.13
Recently, Gardner et al8 described 2 patients with mixed lineage leukemia (MLL) gene-rearranged BCP-ALL with a myeloid CD19– phenotype who relapsed after CART-19 therapy. One of the patients, an infant with MLL-positive BCP-ALL, did not show a clonal IG rearrangement in the myeloid blasts analogous to our index patient, whereas the other patient showed the same clonal IG rearrangement already existing at initial diagnosis, obviously representing a different mechanism.
Similar to that demonstrated by 2 pediatric BCP-ALL cohorts, we demonstrated multilineage involvement of the BCR-ABL1-positive clone.12,14 In the study by Castor et al,12 this finding was restricted to P210BCR-ABL1-positive ALL, but we also demonstrated clonal involvement of the HSC/MPP compartment in patients with P190BCR-ABL1-positive ALL. Thus, characterization of fusion transcripts does not allow a clear assignment to the cell of origin of the BCR-ABL1 fusion even though primarily patients with a major BCR-ABL1 transcript showed the MPP pattern.
In the 2 index patients reported herein, blinatumomab was able to eliminate the aggressive B-cell–determined clone but not the ancestral CD19–BCR-ABL1-positive precursor that gave rise to the CD19– relapse. It seems that the BCR-ABL1 fusion frequently represents an antecedent event in adult BCP-ALL leukemogenesis, which may occur early in the hierarchy of hematopoiesis before B-lineage determination. More frequently than is assumed, BCR-ABL1-positive ALL resembles a chronic myeloid leukemia–like disease in lymphoid blast crisis.14 Therefore, novel therapeutic strategies should target CD19– malignant precursor cells in addition to the B-cell leukemic bulk, especially in patients with the MPP pattern. As an example, Ruella and colleagues described a dual CD19- and CD123-CART approach that prevented antigen-loss relapses in xenograft models.15,16 Our findings also favor a combination with tyrosine kinase inhibitors to target BCR-ABL1-positive cells without CD19 expression. Whether patients with an MPP pattern in their BCR-ABL1-positive ALL show a higher frequency of CD19 escape after application of CD19-directed treatment compared with those with a B-lineage pattern needs to be verified prospectively.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the technical staff of the cytogenetics and molecular cytogenetics laboratories of the Institute of Human Genetics Kiel for their excellent support.
This work was supported by the Deutsche Forschungsgemeinschaft (EXC 306 Inflammation-at-Interfaces CLVII) (D.K. and H.-H.O.), by the KinderKrebsInitiative Buchholz, Holm-Seppensen, and by the Stiftung Leukämie (M. Brüggemann and H.P.).
Authorship
Contribution: M. Bartels, I.N., R.S., and M. Brüggemann conceived and designed the experiments; M. Bartels, I.N., H.-H.O., S.U., A.C., and H.-A.H. performed the experiments; I.N., M. Bartels, J.D., H.B., O.O., H.P., H.T., N.G., D.K., M.K., M.S.T., I.C., R.S., and M. Brüggemann analyzed the data; J.D. and M.S.T. contributed materials; J.D., O.O., H.P., N.G., D.H., and M. Brüggemann provided clinical data; I.N., M. Bartels, H.B., and M. Brüggemann wrote the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: D.K. was on an advisory board for Incysus, Ltd and Qu Biologics, Inc. H.P. received honoraria from Amgen, Novartis, and Jazz Pharmaceuticals and was on an advisory board for Incyte. M. Brüggemann participated in contract research for Amgen, Roche, Affimed, and Regeneron; received honoraria from Amgen, Roche, and Pfizer; and was on an advisory board for Amgen and Incyte. M.S.T. performed research under contract for Amgen, Affimed, and Regeneron and served on an advisory board for Amgen and Regeneron. N.G. performed research under contract for Amgen and Pfizer and received honoraria from Amgen and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Monika Brüggemann, Department of Hematology, University Hospital Schleswig-Holstein, Campus Kiel, Langer Segen 8-10, 24105 Kiel, Germany; e-mail: m.brueggemann@med2.uni-kiel.de.
References
Author notes
I.N. and M. Bartels contributed equally to this study.

![Figure 2. Analysis of relevant immunophenotypic compartments in 27 adult patients with BCR-ABL1–positive BCP-ALL using FISH after FACS. (A) BCR-ABL1 positivity of immunophenotypic compartments in the 2 predominant patterns of BCR-ABL1 occurrence. The green and orange color content of the boxes represents the ratios of BCR-ABL1 fusion–positive and –negative signal constellations observed in the 27 patients. For details of sorting strategies, see supplemental Data. (B) Detailed results of the analysis for each patient and compartment. Green, BCR-ABL1 fusion negative; orange, BCR-ABL1 positive; gray, not analyzable. Overall, 11 of 27 patients showed an MPP pattern, 13 of 27 showed a B-lineage pattern, and 3 of 27 had a pattern between the two (indeterminable [i.d.]). M, major BCR-ABL1 transcript; m, minor BCR-ABL1 transcript; mM, minor and major BCR-ABL1 transcripts identified; n.a., not analyzable.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/18/10.1182_blood-2017-05-782888/4/m_blood782888f2.jpeg?Expires=1769257289&Signature=mMoXEEgXzor8koayx6M3SwAtcUiliPQnSsX6yU0GI3fCyEa9GRNo-4hSALUmC~Mmp8Xwngn~KmuV7JRx6m1m1CiJDN7~RqOxfbBBloguB4ve~MLLrY-5jo-ZWV9OVzBcxlzhIORWs4IKhNN5wyCvBuCgr4KPLTsQVLIpCVbDvs7zw8WOr8uy4MoSFHeYpuYaG5zxZxnfNAVrLZr~AWV6gSovIxApwiRUhAzN3pFJKNjsHhed0aH9Pbnr7fZopO3TzH0U1Gnd08Hoplh6wzxRUWkbX-Y6BAy0pUtGY-oC3LJZP3~iNZ7wKO3YErx38htpSsuiUAm~gNoY6MlAtYdhaA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
