Abstract

Background: Chemoimmunotherapy (CIT) is standard-of-care for patients (pts) with treatment-naïve (TN) CLL. Although outcomes have improved with CIT, targeted therapies that eliminate the need for chemotherapy may be desirable, particularly for older pts or those with high-risk disease. Ibrutinib (ibr), a first-in-class, once-daily inhibitor of Bruton's tyrosine kinase, is approved in the US and EU for the treatment of CLL and allows for treatment without chemotherapy. In the primary analysis of the phase 3 RESONATE-2 (PCYC-1115) study of single-agent ibr vs chlorambucil (Clb) in TN pts ≥65 yrs with CLL, ibr resulted in significantly longer progression-free survival (PFS) with 84% reduction in risk of progression or death and longer overall survival (OS) compared with Clb (Burger, N Engl J Med 2015). We conducted a cross-trial comparison of ibr data with additional follow-up of RESONATE-2 with phase 3 data of CIT regimens in the setting of TN CLL.
Methods: A cross-trial comparison was performed between single-agent ibr therapy in RESONATE-2 (median follow-up 35.7 mo) and CIT regimens from published phase 3 studies conducted in pts with TN CLL: fludarabine + cyclophosphamide + rituximab (FCR) from CLL8 (FCR-CLL8) (Hallek, Lancet 2010), bendamustine + R (BR) and FCR from CLL10 (FCR-CLL10) (Eichhorst, Lancet Oncol 2016), obinutuzumab + Clb (G-Clb) and R-Clb from CLL11 (Goede, N Engl J Med 2014), and ofatumumab + Clb (Ofa-Clb) from COMPLEMENT-1 (Hillmen, Lancet 2015). Data comparisons were limited given the lack of available patient-level data from CIT studies and differences in study design and patient eligibility criteria. Pts with del(17p) were excluded in RESONATE-2 and CLL10. RESONATE-2 enrolled pts ≥65 yrs. CLL8 and CLL10 enrolled pts with low comorbidity (cumulative illness rating scale [CIRS] ≤6 and CrCl ≥70 mL/min). CLL11 enrolled pts with comorbidity (CIRS >6 or CrCl <70 mL/min). COMPLEMENT-1 enrolled pts unsuitable for fludarabine therapy.
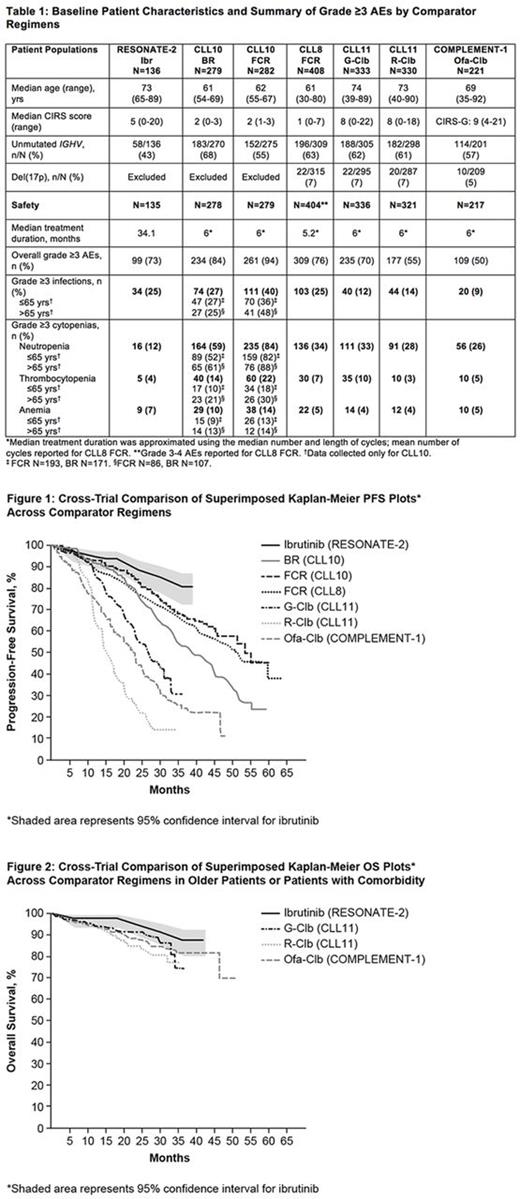
Results: The median age across studies was 61-74 yrs, with older pts enrolled in studies with ibr, G-Clb, or R-Clb (Table 1). Median CIRS scores ranged from 1-9, with lower comorbidity scores for pts treated with BR, FCR-CLL10, and FCR-CLL8 (medians 1-2) (Table 1). Treatment with single-agent ibr was associated with longer PFS compared with CIT regimens (Figure 1). Notably, PFS with ibr compared favorably to CIT studies that also excluded pts with del(17p) (BR and FCR-CLL10), and with CIT studies that enrolled older pts with comorbidities (G-Clb, R-Clb, and Ofa-Clb). Based on comparisons of hazard ratios (HR) for PFS by baseline subgroups from studies that included Clb as the control arm, the magnitude of PFS benefit with ibr vs Clb in high-risk pts including those with unmutated IGHV or del(11q) was greater than those observed with G-Clb or R-Clb vs Clb from CLL11. In pts with unmutated IGHV, PFS HR (95% CI) vs Clb was 0.08 (0.04-0.17) for ibr, 0.23 (0.16-0.34) for G-Clb, and 0.54 (0.38-0.76) for R-Clb. In pts with del(11q), PFS HR (95% CI) vs Clb was 0.02 (0.005-0.11), 0.37 (0.17-0.81) and 0.99 (0.49-2.03) for ibr, G-Clb and R-Clb. Additional PFS comparisons by baseline subgroups will be presented. OS with single-agent ibr appeared favorable to CIT in studies with older or less fit pts (Figure 2). Median treatment duration was 34 mo for ibr and approximately 5-6 mo for CIT regimens (Table 1). The overall rate of grade ≥3 AEs for CIT regimens was highest with FCR, followed by BR and Clb-based regimens. The rate of grade ≥3 AEs was similar for ibr and G-Clb despite the longer AE collection period for ibr (Table 1). The rate of grade ≥3 infections varied by study and ranged from 9% with Ofa-Clb to 40% with FCR-CLL10, and was 25% with ibr. Rates of grade ≥3 cytopenias were generally lower with ibr compared with CIT, including neutropenia (ibr: 12%; CIT regimens: 26% to 84%; Table 1).
Conclusions: Compared with published data of CIT regimens in pts with TN CLL, single-agent ibr was associated with longer PFS and a generally more favorable safety profile despite the longer treatment duration and much longer AE collection period. Acknowledging that interpretation of these results is limited by differences in study design and patient populations, and that conclusions cannot be made on whether ibr is superior to FCR, this cross-trial comparison suggests that ibr may potentially eliminate the need for chemotherapy in some pts with TN CLL.
Robak: Pharmacyclics LLC, an AbbVie Company: Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Akari Therapeutics Plc: Research Funding. Burger: TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Novartis: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding. Tedeschi: Janssen: Consultancy, Other: travel expenses; Gilead: Consultancy, Other: travel expenses; AbbVie: Consultancy. Barr: Novartis: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; Seattle Genetics: Consultancy; Gilead: Consultancy; Infinity: Consultancy; Celgene: Consultancy; AbbVie: Consultancy, Research Funding. Owen: Gilead: Honoraria, Research Funding; Janssen: Honoraria; AbbVie: Honoraria; Celgene: Honoraria; AstraZeneca: Honoraria; Pharmacyclics LLC, an AbbVie Company: Research Funding; Lundbeck: Honoraria; Roche: Consultancy, Honoraria, Research Funding. Bairey: Janssen: Consultancy, Research Funding. Hillmen: Novartis: Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria; GSK: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Pharmacyclics LLC, an AbbVie Company: Honoraria, Research Funding; Celgene: Research Funding. Simpson: Amgen: Research Funding; Onyx: Research Funding; Roche: Honoraria; Pharmacyclics LLC, an AbbVie Company: Research Funding; Celgene: Honoraria, Other: travel expenses. Devereux: Roche: Consultancy, Other: travel expenses; GSK: Consultancy; MSD: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Servier: Other: Advisory board; Gilead: Consultancy, Honoraria, Other: travel expenses, Speakers Bureau; Janssen: Consultancy, Honoraria, Other: travel expenses, Speakers Bureau. McCarthy: Chugai: Other: travel expenses; Celgene: Other: travel expenses; Roche: Consultancy, Other: travel expenses; Janssen: Consultancy, Honoraria, Other: travel expenses; Novartis: Honoraria, Other: travel expenses; AbbVie: Honoraria, Other: travel expenses. Coutre: AbbVie: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Janssen: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; Gilead: Consultancy, Research Funding. Quach: Amgen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Gaidano: Gilead: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Moreno: Gilead: Consultancy, Research Funding; Janssen: Consultancy. Gill: Janssen: Consultancy, Honoraria, Other: travel expenses. Flinn: Pharmacyclics: Research Funding; Pfizer: Research Funding; Forty Seven: Research Funding; Merck: Research Funding; Takeda: Research Funding; Infinity: Research Funding; Acerta: Research Funding; Gilead: Research Funding; TG Therapeutics: Research Funding; Calithera: Research Funding; Portola: Research Funding; Incyte: Research Funding; Pharmacyclics LLC: Research Funding; AbbVie Company: Research Funding; Trillium: Research Funding; Genentech: Research Funding; Janssen: Research Funding; Curis: Research Funding; KITE: Research Funding; Constellation: Research Funding; Verastem: Research Funding; Beigene: Research Funding; Seattle Genetics: Research Funding; Janssen: Research Funding; Celgene: Research Funding; Novartis: Research Funding; Agios: Research Funding. Gribben: Gilead: Other: travel expenses; Acerta: Research Funding; Janssen: Research Funding; Celgene: Consultancy; Unum: Consultancy. Mokatrin: Pharmacyclics LLC, an AbbVie Company: Employment; AbbVie: Equity Ownership. Cheng: Pharmacyclics LLC, an AbbVie Company: Employment; AbbVie: Equity Ownership; Johnson & Johnson: Equity Ownership. Styles: AbbVie: Equity Ownership; Pharmacyclics LLC, an AbbVie Company: Employment. James: Pharmacyclics LLC, an AbbVie Company: Employment; AbbVie: Equity Ownership. Kipps: Genentech: Consultancy, Research Funding; Gilead: Consultancy, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding; Oncternal: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria. Ghia: Pharmacyclics LLC, an AbbVie Company: Consultancy; Janssen: Consultancy, Research Funding; Gilead: Consultancy, Research Funding, Speakers Bureau; Adaptive: Consultancy; AbbVie: Consultancy; Roche: Consultancy; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract