Abstract
Background
Systemic AL amyloidosis (AL) is associated with an underlying plasma cell dyscrasia and caused by deposition of abnormally folded monoclonal immunoglobulin light chains. Novel chemotherapy has improved overall survival (OS) and haematological responses in recent years but little is known about durability of treatment response in this disease. In multiple myeloma, progression free survival (PFS) without ASCT is short. We report data on PFS and time to next treatment (TNT) in a predominantly non-ASCT population from the ALChemy study, a large prospective study of patients with AL.
Methods
All patients with newly diagnosed AL amyloidosis from January 2009 to March 2016 were included. All underwent serial organ function assessment, imaging and biomarkers. Organ involvement and responses were defined by international amyloidosis consensus criteria. Survival was calculated by Kaplan-Meier analysis. All outcomes are reported on an intention-to-treat (ITT) basis (patients who died before response assessment were assessed as non-responders). Progression free survival (PFS) was defined as time to death or progression to next line of chemotherapy. Time to next treatment (TNT) was defined as time from the beginning of first line therapy to the next line.
Results
1203 patients were recruited. Median age was 66.3 years (29.5-89.4), M:F 58%:42%, median NT-proBNP was 1974ng/L and median dFLC was 181.7mg/L. 62 patients (5.2%) died before treatment initiation. Treatments were: bortezomib-based regimen (mainly CyBorD) - 57.6%, immunomodulatory (IMID)-based (mainly CTD) - 32.4%, oral melphalan-dexamethasone - 5%, rituximab-based regimen - 3.2%, upfront ASCT - 1.3%, other - 0.5%. Median number of chemotherapy cycles was 5. Overall haematological response (FLC and M-protein response) at 6 months on an ITT basis (n=1037) was: CR 17.6%, VGPR 15.3%, PR 17.7% and non-response 49.4%. On an evaluable basis, overall haematological response at 6 months (n=723) was: CR 25.3%, VGPR 22%, PR 27.1% and non-response 25.6%.
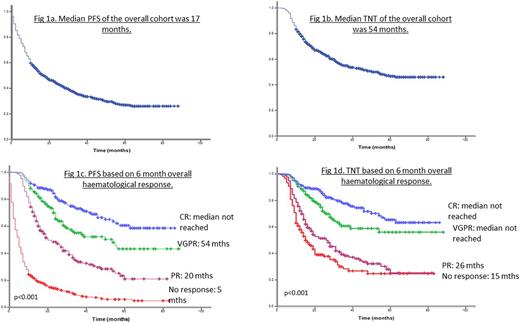
260 patients (21.6%) died within 6 months of diagnosis. Median OS was 47 months. Median PFS and TNT of the whole cohort was 17 months and 54 months respectively (Fig 1a and 1b). Median TNT in Mayo Stage 1, 2, 3A and 3B was 44, 46, 48 months and not reached (no second line treatment by 5 years = 54%), (p=0.63). Median PFS in Mayo Stage 1, 2, 3A and 3B was 34, 26, 14 and 4 months respectively (p<0.001).
Median OS was not reached in patients who achieved an overall PR, VGPR or CR at 6 months, compared to median OS of 8 months in non-responders (p<0.001). Median PFS in patients who achieved an overall CR, VGPR, PR or non response at 6 months was: not reached (5 year PFS = 77%), 54 months, 20 months and 5 months respectively (p<0.001) (Fig. 1c). Median TNT in patients in overall CR, VGPR, PR or non response at 6 months was as follows: not reached (66% at 5 yrs), not reached (55% at 5 yrs), 26 months and 15 months (p<0.001) (Fig. 1d).
Median OS in patients treated with bortezomib, IMID and melphalan was: 83 months, 55 months and 42 months respectively (p=0.11). Median OS in Stage 3 patients treated with bortezomib and IMID was 38 and 12 months respectively (p=0.001). Median PFS in patients treated with bortezomib, IMID and melphalan was: 21, 15 and 14 months (p=0.04). Among patients with stage 3 disease, median PFS in patients treated with bortezomib and IMID was 15 and 7 months respectively (p<0.001). Median TNT in patients treated with bortezomib, IMID and melphalan was: not reached (no second line by 5 years = 57%), 32 and 26 months (p=0.001). Among Stage 3 patients, median TNT in patients treated with bortezomib and IMID therapy was not reached (no second line by 5 years = 63%) and 28 months respectively (p<0.001).
Conclusion
These prospectively collected data from a real world setting report on progression free survival and time to next treatment in AL with predominant non-transplant novel agent based regimens, which has thus far been lacking. Patients treated with bortezomib based therapy had superior PFS, TNT and OS. Median TNT was 4.5 years. Strikingly, PFS and TNT were remarkably long in patients achieving a complete haematological response (PFS 77% at 5 years) - akin to those previously reported in patients treated with upfront ASCT and perhaps raising questions about the role of upfront ASCT. These data confirm the efficacy of bortezomib based regimens in AL amyloidosis and the value of CR as the goal of induction therapy in this disease.
Wechalekar: Celgene: Honoraria; Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.