Abstract
Recent advances in the field of haploidentical hematopoietic stem cell transplantation (Haplo-SCT) allowed larger access to allogeneic treatment in absence of HLA-matched donor. Indeed, former limitations associated with HLA disparity could be overcome by the use of post-transplant cyclophosphamide (PT-Cy) as part of GVHD prophylaxis. Low toxicity observed after PT-Cy Haplo-SCT enabled its diffusion to older patients who are disproportionally affected with hematological malignancies. The aim of our study was to assess the outcome after Haplo-SCT in this specific setting of elderly patients.
Inclusion criteria were: age >= 60 years; first Haplo-SCT for hematological malignancy at 2 institutions from 2011 to 2016; PT-Cy as part of GVHD prophylaxis. All types of graft source and conditioning regimens were allowed except sequential conditioning regimen.Geriatric assessment was performed previous Haplo-SCT by geriatric oncologist for patients aged of 65 years or more.One hundred and twenty-one consecutive patients who met inclusion criteria were included.
Median age was 66 years (range: 60-75) whereas 23 patients (19%) were 70 years or more. Eighty-two patients were transplanted for myeloid malignancies (68%). Forty-eight patients (39%) had active disease at time of transplantation.Hematopoietic cell transplantation comorbidity index (HCT-CI) was >= 3 in 70 patients (58%) and disease risk index (DRI) was high or very high in 36 patients (30%). Conditioning regimens were based on reduced doses of busulfan (RIC; N=70; 58%) or on low dose TBI (NMAC; N=51; 42%).Peripheral blood stem cells were the most frequently used graft source (N=108; 89%). Ninety-four patients (78%) were transplanted using a child as donor. Fifteen patients received prophylactic donor lymphocyte infusion (DLI) in a median time of 109 days after transplant, as defined by full donor chimerism and no evidence of relapse. Median follow-up was 20 months (range: 6-58).
Cumulative incidences of grade II-IV, grade III-IV acute GVHD and moderate to severe chronic GVHD were 23%, 10% and 10 % respectively. 3 patients developed GVHD after DLI. At 2 years, the cumulative incidence of non-relapse mortality (NRM) and relapse were 26% and 23 %, respectively.
The 2-year overall survival, progression free survival, and survival with no relapse and no steroid-requiring chronic GVHD were 57%, 52%, and 43 %, respectively. Interestingly, HCT-CI was not able to predict differential outcome in this very specific setting of Haplo-SCT for elderly patients (2-year NRM: 0-2 vs. >=3: 21% vs. 29%, p=0.32).
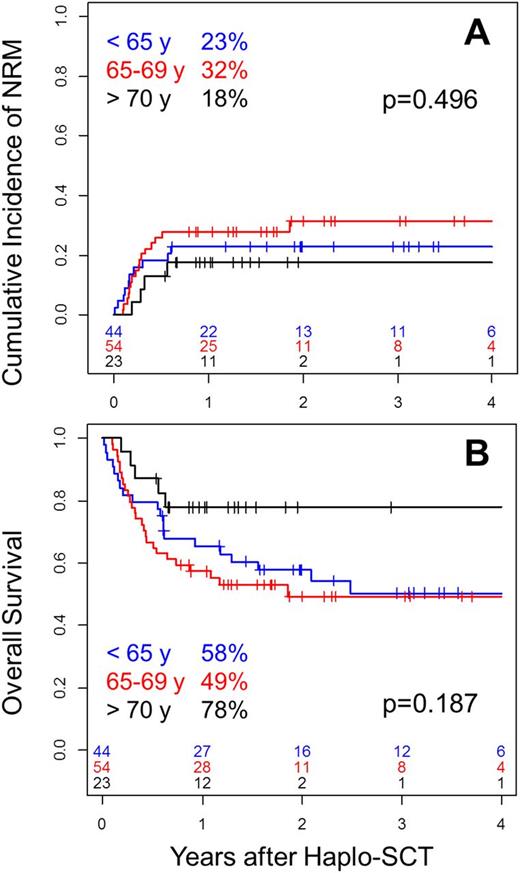
We did not found higher transplantation-related morbidity and mortality in patients aged of 70 years or more. Indeed, day-100 grade II-IV acute GVHD (<70 vs. >=70: 26% vs. 13%, p=0.12), 2-year moderate + severe chronic GVHD (<70 vs. >=70: 8% vs. 20%, p=0.15) and 2-year NRM (<70 vs. >=70: 27% vs. 18%, p=0.36Figure 1A) were not significantly increased in older patients. The very promising outcome in patients >= 70 years of age (PFS: 73%; OS 78%, Figure 1B) supports that a cautious selection of oldest patients allows achieving low toxicity using adequate preparation, mostly NMAC regimens.
We can conclude that Haplo-SCT is a valid approach for elderly patients. While HLA disparity was initially considered as a limitation to transplant elderly patients, Haplo-SCT has finally appeared as a particularly well-adapted alternative in this population. The low incidences of GVHD (less than 10% of severe forms) using a PT-Cy platform could explain in part this specific observation and may challenge the use of unrelated donor which is associated with high incidences of GVHD, especially in older patients. Our study underlines that Haplo-SCT using PT-Cy can provide a low-toxicity allogeneic platform for elderly patients, based on which early active post-transplantation strategies should be developed for better disease control.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.