Abstract
BACKGROUND: Treatment of disease relapse after alloHCT is inadequate. We previously reported that checkpoint blockade with ipilimumab (ipi) at 10 mg/kg induced responses in 32% of patients (pts) with relapsed hematologic malignancies (HM) after alloHCT, whereas no responses were noted at 3 mg/kg (Davids et al., N Eng J Med, 2016). Here, we update the published data on the 10 mg/kg cohort with additional follow-up. We also report for the first time on two new cohorts: 1) a cohort dosed at ipi 5 mg/kg, chosen to explore the balance of efficacy and toxicity at this intermediate dose and 2) a nivolumab (nivo) cohort to propsectively evaluate this approach, in light of recent retrospective data suggesting that anti-PD1 antibodies are active but may also have significant toxicity in this population (Herbaux et al. and Haverkos et al., Blood, 2017).
METHODS: The primary objectives for all cohorts in this phase I/Ib, multicenter, investigator-initiated, CTEP-sponsored study (CTEP 9204) were to determine MTD and evaluate safety. Secondary objectives were to assess efficacy and immunologic correlates. Ipi induction was given IV q3 wks for 4 cycles, followed by maintenance q12 wks for up to 1 year. Nivo was given at a starting dose of 1 mg/kg q2 wks until time of progression or unacceptable toxicity. Disease-specific response evaluations for ipi pts were performed at the mid-point (7 wks), end of induction (13 wks), and during maintenance, and for nivo pts q4 cycles (q8 wks).
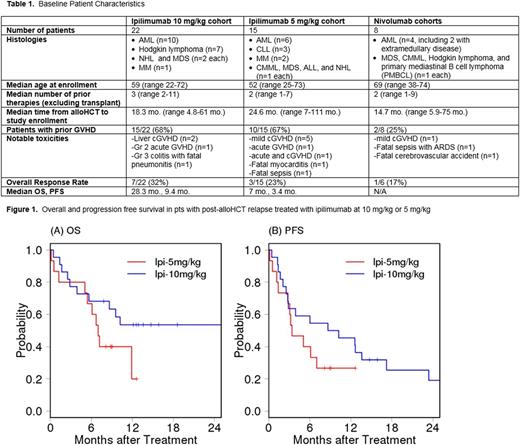
RESULTS: In the original 22 pts in the ipi 10 mg/kg cohort (Table 1), now with a median follow-up of 15 mo., 9 pts remain alive, 4 of whom are progression free. Ongoing response has been seen in 2 of the 3 pts with leukemia cutis (30 and 32 mo. in CR), 1 pt with AML (34 mo. in CR), and 1 pt with MM (39 mo. in remission) who was previously reported as PR but achieved CR 18 mo. after initial dosing. The median OS and PFS are 28.2 and 9.4 mo., respectively (Figure 1). GVHD occurred in 3/22 (14%) pts, including mild liver cGVHD (n=2) and gr2 acute GVHD of the gut (n=1). Immune-mediated events occurred in 1 pt each with gr2 ITP, gr2 pneumonitis, and gr3 colitis and fatal pneumonitis.
In a phase Ib expansion cohort, 15 additional pts were treated at ipi 5 mg/kg. Six pts developed GVHD on study (5 mild chronic, 1 gr I acute). Immune-related adverse events (irAEs) included: fatal myocarditis after the first dose of ipi (n=1), post-infusion gr4 fever (n=1), gr4 AIHA (n=1), gr3 ITP (n=1), and gr2 biopsy-proven non-GVHD colitis (n=1). One pt died due to sepsis <1 wk after initial dosing. In the 13 pts evaluable for efficacy, 3 (23%) achieved partial response (MM (n=2) and MDS (n=1)). The pt with MM who achieved VGPR remains in remission > 1 year after initial dosing. A pt with CLL who did not meet formal response criteria had a 46% and 50% reduction in lymph node and marrow disease, respectively. With a median follow-up of 9 mo, the median OS and PFS are 7 and 3.4 mo., respectively (Figure 1).
In the nivo cohort, 8 pts have been treated to date, 6 pts at 1 mg/kg and 2 pts at 0.5 mg/kg. The median number of cycles given to date is 4 (range 1-14). In the 1 mg/kg cohort, 1 pt experienced cGVHD (mild myofascial). Two irAEs resulted in DLTs, including one pt with sepsis and fatal ARDS, and one pt who developed autoimmune pancytopenia, anti-phospholipid antibodies, and fatal thrombotic cerebral vascular accidents. Other irAEs included gr3 pneumonitis and transaminitis (n=1 each). In a preliminary efficacy analysis, a pt with primary mediastinal B cell lymphoma achieved PR and remains in remission over 8 mo. after starting therapy. Notably this was the pt who experienced mild myofascial cGVHD. Due to the toxicity observed at 1 mg/kg, a 0.5 mg/kg cohort recently opened, and no significant toxicities have been observed in the 2 pts accrued to date.
CONCLUSION: Checkpoint blockade can provide durable CR in a subset of pts with relapsed HM after alloHCT, with a median OS of 28.2 mo. in pts treated with ipi 10 mg/kg. At ipi 5 mg/kg, responses were also observed, but this dose did not appear to improve the rate of GVHD or immune-mediated toxcities. We also report early data from our nivo cohort, the first prospective clinical trial of an anti-PD1 antibody for relapsed HM post-alloHCT. Although GVHD has been uncommon to date in the nivo cohort, serious irAEs occurred, highlighting the importance of dose exploration and prospective evaluation in a clinical trial setting. Accrual continues to the nivo cohort of this ongoing study (NCT01822509).
Davids: Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy; Infinity: Consultancy, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; InCyte: Membership on an entity's Board of Directors or advisory committees; Astra-Zeneca: Consultancy; Merck: Consultancy; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees. Costello: Janssen: Honoraria, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Herrera: BMS: Consultancy, Research Funding; Seattle Genetics: Research Funding; Immune Design: Research Funding; Merck: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding. Locke: Cellular Biomedicine Group Inc: Consultancy; Kite Pharma: Consultancy. Avigan: Genus Oncology: Research Funding. Cutler: Pfizer: Consultancy; Pharmacyclics: Consultancy; Kite: Consultancy; Incyte: Consultancy; Bristol-Myers Squibb: Consultancy; Astellas: Consultancy. Koreth: Prometheus Labs: Research Funding; Kadmon Corp: Membership on an entity's Board of Directors or advisory committees; Amgen Inc.: Consultancy; Takeda Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Millennium Pharmaceuticals: Research Funding. Wu: Neon Therapeutics: Consultancy. Armand: Affimed: Research Funding; Sigma Tau: Research Funding; Sequenta/Adaptive: Research Funding; Merck & Co., Inc.: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Otsuka: Research Funding; Infinity: Consultancy; Genmab: Consultancy; Roche: Research Funding; Tensha: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.