Abstract
Background
Vitamin D (VD), due to its interaction to vitamin D receptor (VDR), has a potent immunomodulator effect. Some retrospective studies have shown that VDR polymorphisms (which determine the affinity of VDR towards VD) can influence on the outcome and the incidence of GVHD after allo-HSCT. We have recently described in a phase I/II prospective trial (ALOVITA) that the use of VD in the post-transplant setting decreases the risk of overall and moderate-severe chronic GVHD (Caballero-Velazquez, Clin Cancer Res 2016).
Objective
To analyze the effect of VDR polymorphisms on post-transplant outcomes and on the effect of VD on cGVHD incidence in patients included in the ALOVITA clinical trial.
Methods
Patients and donors with genomic DNA, extracted from peripheral or bone marrow blood prior to transplant, available were analyzed. A group of patients who received 1000 UI/day or 5000 UI/day of VD (n=71), and a control group (n=36) were included. Overall, 107 patients and 93 donors were selected. The polymorphisms FokI C>T, BsmI A>G, ApaI T>G, TaqI T>C were detected by TaqMan SNP genotyping assay.
Results
Baseline characteristics of patients are shown in table 1.
The median follow-up was 453 days (13-1256d). The frequencies of genotypes coincided with that of the European population as described in international databases.
There were no statistically significant differences in terms of NRM depending on polymorphisms for FokI, BsmI ApaI or TaqI neither in patients nor in donors, although a trend towards a decreased NRM was observed in patients whose donors were ApaI GG (10%) as compared to TG+TT (27.8%), (p=0.07).
A higher relapse incidence (RI) was observed in patients with TaqI CC genotype (42.9%) vs those with TC+TT genotype (20.6%), p=0.02 as well as in patients with BsmI GG (37.5%) vs AA+GA (21%), p=0.04. Also, patients whose donors had an ApaI GT+TT genotype had a higher RI (26,8) as compared to GG (7,1), p=0.05.
A trend towards a better EFS was observed for BsmI AA in donors (75.6%) vs GA+GG (48.7%), p=0.06. Regarding OS, significant differences were observed depending on the genotype of the patients for FokI: CT (84%) vs CC+TT (62,1%), p=0.03. .
We analyzed BsmI, ApaI and TaqI haplotypes in 95 patients. The most frequent combination within our series were AAGGTT (group 1: n=23) and GATGTC (group 2: n=23). We named 'group 3' to include the rest of the combinations (n=49). We observed significant differences in terms of OS: 73% vs 51.1% vs 81.6% for groups 1, 2 and 3, respectively (p=0.02).
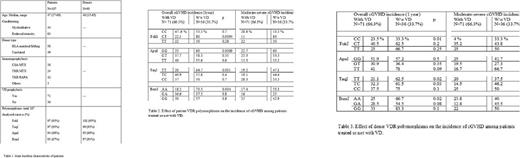
As far as cGVHD is concerned, data are shown in Table 2/3. The following patient genotypes influenced on the efficacy of VD as cGVHD prophylaxis, the incidence of overall cGVHD being: FokI, CT (22.5% vs 80%, p=0.0004); ApaI, GG (23% vs 60%, p=0.009); TaqI, TT (20% vs 64.7%, p=0.001), BsmI, AA (18.2% vs 73.3%, p=0.0004) for those patients receiving or not VD. These differences were also observed for moderate-severe cGVHD. The same analysis was performed for donors' genotypes, and differences in overall and moderate-severe cGVHD were observed for the following genotypes: FokI, CC; ApaI, TT; TaqI, TC; BsmI, AA, (table 2).
We also evaluated the benefit of GVHD prophylaxis with VD regarding patients' haplotype. Group 1 haplotype had the greatest benefit from receiving VD in terms of cGVHD: overall cGVHD: 75% vs 15.4% for patients who did and did not receive VD, p=0.008, and moderate-severe cGVHD: 75% vs 14.3%, respectively; p=0.01.
Conclusion:
Patient VDR polymorphisms influence on outcome after transplantation. The VDR genotypes from either the donor or the patient modulate the effect of VD used post-transplant on the risk of cGVHD, thus suggesting that genomic changes in VDR can modify its interaction to VD.
Sanchez-Guijo: Incyte: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.