Abstract
Background
B-cell prolymphocytic leukaemia (B-PLL) is a rare disorder representing 1% of lymphocytic leukemias. With a median age of 69 years, pts characteristically present with B symptoms, marked lymphocytosis, cytopenias, massive splenomegaly and minimal lymphadenopathy. The largest trial of B-PLL included only 14 patients (pts) (Döhner et al, 1993). There are no randomized trials published in B-PLL. The median overall survival is 3 years. 13q14, 11q23 and 17p deletions are common by fluorescence- in-situ hybridisation (FISH) (Lens et al, 1999). TP53 mutations are noted in >50%, conferring resistance to immunochemotherapy (Dearden 2012). In the absence of international guidelines or trial data, clinicians typically employ fludarabine, cyclophosphamide and rituximab (FCR) or bendamustine-R (BR) in TP53- intact pts with allogeneic stem cell transplant (allo-SCT) consolidation in eligible, chemotherapy-sensitive pts (Castagna et al, 2005). For TP53 -disrupted B-PLL, alemtuzumab has efficacy but is associated with serious infectious toxicity.
The B-cell-receptor signaling pathway plays a critical role in CLL pathogenesis, although little is known regarding its importance in B-PLL (Bernal et al, 2001). This pathway is partly mediated by the activation of the delta isoform of phosphatidylinositol 3-kinase (PI3Kδ) which is highly expressed in lymphoid cells (Herishanu et al, 2011). Idelalisib is a first-in-class, potent, oral, selective PI3Kδ inhibitor. Initial results of the first 5 pts in the compassionate use programme describe clear efficacy of idelalisib-rituximab (I-R) in TP53- disrupted B-PLL (Eyre et al, 2016). We update this series with considerably longer follow up and additional pts.
Methods
8 pts received I-R in a UK-wide compassionate use programme supported by Gilead. Data was retrospectively collected for adverse events (AEs), clinical and radiological responses. Rituximab (375 mg/m2) was given 2-weekly (cycle (C) 1-2) and then 4-weekly (C3-6). Idelalisib 150 mg b.d. was given continuously with dose interruption and reduction as required for toxicity and was continued in pts with response after C6 as monotherapy. Response was ascertained radiologically and clinically according to institutional practice. AEs were reported prospectively to Gilead. FISH for p53 deletion was performed in all pts. Sanger sequencing was performed in 3 pts. Standard infectious prophylaxis was routinely added at the time that the safety signal was announced.
Results
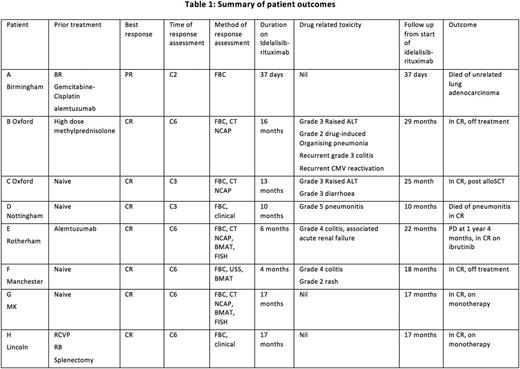
The median age was 64.5 years (range: 57-76 years). 7 were male and 1 was female. The median time from diagnosis to first treatment was 9.5 months (range: 2-60 months). 1 pt had a prior splenectomy, but all others had small volume lymphadenopathy and splenomegaly prior to I-R. TP53 deletions by FISH were found in 7 pts. No known additional mutations were noted. Standard cytogenetics were not performed. 4 pts were treatment naïve, 1 pt received prior methylprednisolone, 1 pt BR, gemcitabine-cisplatin and alemtuzumab, 1 pt alemtuzumab and 1 pt R-CVP and then BR. 5 pts completed 6C x 28-day I-R. 3 pts stopped early: 1 pt died of an unrelated lung cancer in C2, and 2 pts stopped due to idelalisib-related grade (G) 4 colitis at C4.
All 8 pts responded to I-R. The single pt who died early of lung cancer obtained a partial response. All other 7 pts obtained complete remission (CR) when assessed by examination, FBC, radiological and FISH. Of those 7 pts, only 1 progression has occurred. This pt subsequently responded to ibrutinib. 1 pt (treatment naïve) died in CR of G5 pneumonitis at 10 months on therapy. There are 5 ongoing CRs at a median of 18 months (range 17-29 months). 2 of these 5 have stopped idelalisib due to toxicity (both G3-4 colitis), 2 remain on monotherapy, and 1 has undergoing consolidation with an allo-SCT having stopped idelalisib (G3 diarrhoea). The median follow up for all pts is 18 months (range 1-29 months).
Conclusions
I-R displays rapid, deep and durable remissions in TP53 -disrupted B-PLL. The toxicity profile of idelalisib is well described and is clearly demonstrated in this cohort. Pneumonitis, colitis and CMV reactivation were particularly noteworthy. Of interest, remissions were durable in the 2 pts that stopped therapy following colitis for 13 and 14 months respectively. Our data supports importance of compassionate use programme to study novel agents in rare disorders. Updated response and toxicity data will be presented.
Fox: Gilead: Consultancy, Honoraria, Other: travel expenses, Speakers Bureau. Bloor: Roche: Honoraria; Gilead: Consultancy, Other: travel expenses; AbbVie: Honoraria, Other: travel expenses; Janssen: Other: travel expenses, Speakers Bureau. Dungawalla: Gilead: Honoraria. Shankara: Gilead: Honoraria, Other: Travel expenses, Speakers Bureau. Schuh: Abbvie: Honoraria; Janssen: Honoraria; Abbvie: Honoraria; Gilead: Honoraria, Research Funding; Roche: Honoraria; Novartis: Honoraria; Novartis: Honoraria; Celgene: Honoraria; Gilead: Consultancy; Roche: Honoraria; Janssen: Honoraria; Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.