Abstract
Background: Primary central nervous system lymphoma (PCNSL) is a rare type of lymphoma typically associated with aggressive clinical course and poor outcome. Most cases of PCNSL are diffuse large B-cell lymphoma (DLBCL). Induction regimens incorporating CNS penetrating doses anti-metabolite containing induction therapy and high-dose chemotherapy with autologous stem cell transplant have improved outcomes for patients with primary CNS DLBCL in phase II clinical trials (Ferreri et al, Lancet Haem 2016; Omuro et al, Blood 2015). However, due to the rarity of non-DLBCL PCNSL, the outcomes of these patients are not well described.
Patients and Methods: Surveillance, Epidemiology and End Results (SEER) database was used to evaluate survival outcomes among adult (≥18 yrs) patients diagnosed with PCNSL between 1998 and 2014. We only included patients with stage I disease in this analysis. Overall survival (OS) was calculated from diagnosis to death from any cause using the Kaplan-Meier method. Differences in the survival function in different groups were analyzed using the log-rank test. Cox proportional hazards models were used to evaluate associations between patient characteristics and survival.
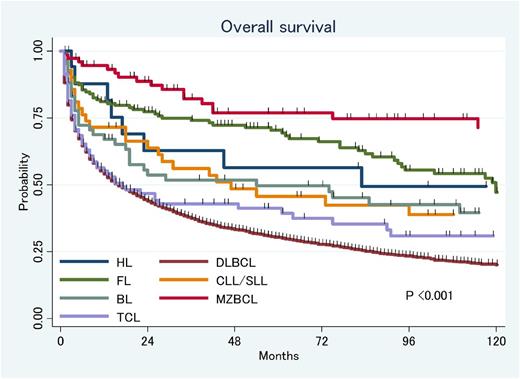
Results: A total of 4375 patients were diagnosed with stage IE PCNSL. The median age of the patients was 64 years (range, 18-96 years). DLBCL was the most common histology (N=3,091), following follicular lymphoma (FL, N=83), peripheral T-cell lymphoma (PTCL, N=64), marginal zone lymphoma (MZL, N=63), Burkitt lymphoma (BL, N=27), small lymphocytic lymphoma (SLL, N=22) and Hodgkin lymphoma (HL, N=13). 1,012 patients (23%) were reported with other or unclear histology. With a median follow up of 43 months, 3,083 patients (70%) died and the median OS was 18 months (95%CI: 16-19 months). Twenty-two percent of patients received neither radiation therapy (RT) nor chemotherapy, 19% of patients received RT alone, 40% of patients received chemotherapy alone and 19% of patients received combined modality therapy (CMT). Receiving chemotherapy was associated with significantly longer OS relative to patients who received RT alone in aggressive lymphoma such as DLBCL, PTCL, HL and BL but RT alone showed similar survival outcome compared to chemotherapy or CMT in FL, SLL and MZL. There is a significant difference in OS by histology (Figure): 5-year OS was 30% in DLBCL, 66% in FL, 33% in PTCL, 79% in MZL, 42% in BL, 38% in SLL and 45% in HL. Multivariate analysis adjusted with age and treatment confirmed that FL and MZL have significantly longer survival compared to DLBCL.
Summary: PCNSL is difficult to treat; however, outcomes vary considerable among different histologic subtypes, with DLBCL and PTCL having the poorest outcome. Acknowledging probable treatment allocation bias, patients with FL and MZL could be treated with RT alone.
Fanale: GENENTECH: Research Funding; ONYX: Research Funding; CELGENE: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; TAKEDA: Honoraria, Research Funding; MOLECULAR TEMPLATES: Research Funding; ADC THERAPEUTICS: Research Funding; AMGEN: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; SEATTLE GENETICS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MERCK: Membership on an entity's Board of Directors or advisory committees, Research Funding. Nastoupil: Karus Therapeutics: Research Funding; TG Therapeutics: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Gilead: Honoraria. Westin: Celgene: Membership on an entity's Board of Directors or advisory committees; Apotex: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees. Neelapu: Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Karus: Research Funding; Bristol-Myers Squibb: Research Funding; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Poseida Therapeutics, Inc: Research Funding; Cellectis Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract