Abstract
Background
Microenvironmental niches within the bone marrow and lymph node have been shown to play a significant role in promoting CLL cell survival and proliferation. Several intracellular signalling pathways have been identified as key mediators of the interaction between CLL cells and accessory cells within these tissues. A better understanding of the underlying biology of the CLL cell and the roles of the tumor microenvironment have provided the rationale for trials of a range of novel therapeutic agents including ibrutinib and idelalisib, which target components of the BCR signaling pathway [Brown et al. Blood 2014, Byrd et al. Blood 2014]. Curative approaches for CLL therapy must target the proliferative, drug-resistant compartments of disease within these microenvironments.
The NanoString® nCounter platform enables mRNA profiling of archival samples, including formalin fixed, paraffin embedded tissue (FFPE). A recent study from our laboratory demonstrated the utility of this technology in profiling lymph node and bone marrow sections from patients with diffuse large B-cell lymphoma for determining cell of origin classification [Gifford et al. BJH 2017].
We have employed the NanoString® technology to profile and compare the mRNA expression profile of CLL cells derived from the peripheral blood, bone marrow and lymph node. In addition, we profiled PB-derived CLL cells following in vitro co-culture with a human stromal cell line under either normoxic or hypoxic conditions, mimicking these tissue microenvironments.
Methods
RNA was extracted from FFPE archival bone marrow trephines (n = 2) and lymph node sections (n = 2), using the QIAGEN RNeasy FFPE Kit. All biopsies analysed were comprised of > 80 % lymphocytes, as determined by microscopic review.
RNA from cryopreserved PBMC fractions (n = 4) was isolated either immediately following thawing or following co-culture with HS5 stromal cells for 24 h under normoxic (n = 4) or hypoxic (n = 4) conditions using the QIAGEN RNeasy Mini Kit.
RNA from all preparations was quantified using a NanoDrop™ spectrophotometer. A total of 200ng of FFPE-derived RNA and 100ng of PBMC-derived RNA was analysed per sample on the NanoString® platform using a 260 gene panel comprised of the Human Cancer Reference gene panel (NanoString) spiked with an additional 30 genes of interest.
Two-fold increases or decreases in mRNA expression were considered significant.
Results
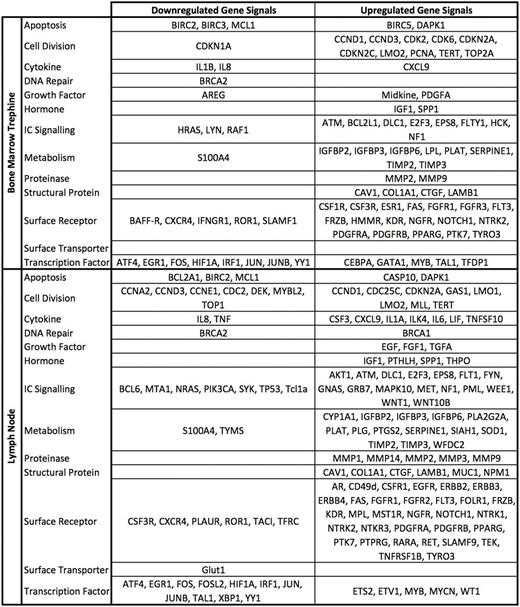
Of the 260 genes profiled, 107 were upregulated in the lymph node samples and 68 in the bone marrow samples compared to expression in peripheral blood-derived CLL cells. Changes were seen in genes encoding surface receptors such as EGFR and NOTCH1, genes coding for intra-cellular signalling proteins such as ATK and WNT and genes associated with metabolism and cell division. 40 genes were downregulated in the lymph node samples and 27 in the bone marrow samples. These changes were seen in genes coding for surface receptors such as BAFF-R and CXCR4, genes coding for intra-cellular signalling proteins such as SYK and PI3K and genes coding for transcription factors such as JUN and FOS.
Co-culturing PBMCs with an HS5 stromal layer induced similar changes to those observed in our comparison of the PBMCs and trephine samples; 56.8% and 44.7% of the mRNA expression changes observed in the bone marrow were observed in PBMCs cultured under normoxic and hypoxic conditions respectively. A similar comparison of the lymph node samples identified concordant changes in expression of 46.2% and 36.8% of genes under normoxic and hypoxic conditions respectively. Importantly, changes observed in genes coding for the anti-apoptotic protein MCL1, the surface receptors CXCR4 and ROR1 and the transcription factors ATF, FOS and JUN were consistent across samples from lymph nodes, marrow and the in vitro model.
In summary, our data demonstrate that it is possible to of assess mRNA expression levels in FFPE archival tissue from CLL patients using the NanoString® platform. We have utilised this technology to profile PB, bone marrow and lymph node derived CLL cells and have identified a number of genes that are either up or down-regulated in cells derived from these microenvironments or following culture in an in vitro model of the tumour microenvironment. These data increase our understanding of how CLL cells populate and proliferate in the tumour microenvironment and lead to therapeutic strategies that target these cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.