Abstract
Background:
Acute myeloid leukemia (AML) is a heterogenous disease with variable outcome depending on cytogenetic as well as molecular risk at presentation. 70-80% of AML patients would achieve complete remission after induction chemotherapy. However, more than half would suffer a relapse. Currently there is no specific test available in clinical practice to detect early relapse. Relapse is usually suspected when there is a change in peripheral blood count or rising LDH. This often proven to be a frank relapse disease by bone marrow examination. A sensitive and specific biomarker in plasma to detect early disease relapse is not available. In this study, we aimed to identify a new biomarker in AML plasma that could potentially be used in clinical practice for detection of early disease relapse.
Method:
Plasma samples were obtained from 8 newly diagnosed AML patients. Patients with fever, renal impairment, heart failure, and abnormal liver function test were excluded to reduce background noise in proteomic analysis. These 8 samples were than matched with 8 normal blood donors plasma that were age and gender matched. Plasma samples would be processed for iTRAQ (isobaric tagging for relative and absolute quantification) and analysed. 1 mL of plasma sample would be delipidated and 7 of the most abundant proteins removed using the MARS Hu-7 affinity column (Agilent Technologies, USA) to prevent the masking of low abundance proteins. Samples comprising 100mg of proteins were then reduced, alkylated, digested and labeled with iTRAQ reagents that differ by 1 atomic mass unit. Protein identification and relative quantification with statistical analysis are performed using in-house QSTAR Elite Q-TOF LC/MS/MS mass spectrometer coupled with ProteinPilotTM software (version 2.1). Proteins that were overexpressed in AML patients, which has potential relation with leukemogenesis would be shortlisted and further quantified in 42 other AML patients and 40 normal blood donor plasma for validation. Correlation with clinical data were carried out.
Results:
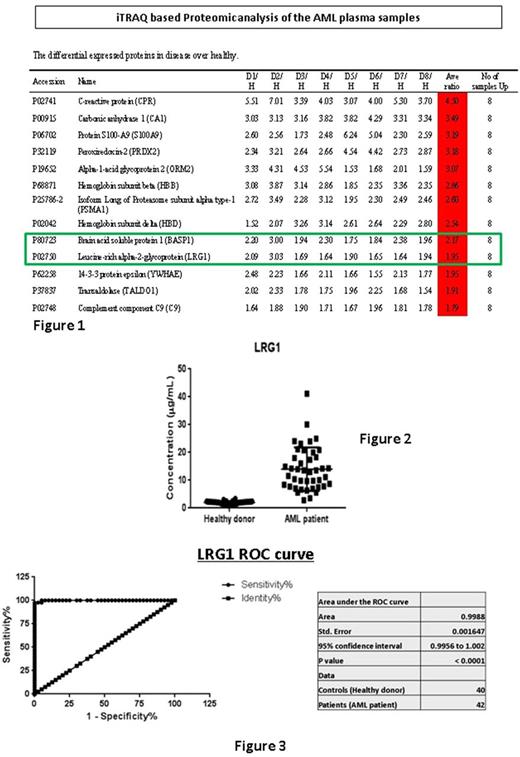
13 proteins were found to be overexpressed in all 8 AML samples with an average expression ratio ranging from 1.75 to 4.50 (see figure 1). Two proteins were shortlisted for its relation with haematopoietic cell - Brain acid soluble protein 1 (BASP1) and Leucine-rich alpha-2-glycoprotein (LRG1). Further validation in normal plasma and AML plasma revealed that BASP1 protein did not have sufficiently good sensitivity and specificity. BASP1 mean concentration in normal plasma was 8.05 ng/mL (SD: 6,29 ng/mL), and in AML samples was 4.41 ng/mL (SD:1.26 ng/mL). In contrast, LRG1 protein showed a significant difference in concentration. LRG1 mean concentration in normal plasma was 2.03 μg/mL (SD: 0.49 μg/mL), compared to AML samples - 13.29 μg/mL (SD: 7.80 μg/mL), p<0.001 (see figure 2). ROC analysis of LRG1 protein for differentiating AML samples from normal plasma sample revealed an AUC of 0.998 (see figure 3), p<0.001. Cut-off level of 3.17 μg/mL would give a sensitivity level of 97.6% (95%CI: 87.4-99.9%), and specificity level of 97.5% (95%CI: 88.8-99.9%). Correlation with clinical data revealed significant correlation with total white cell count at diagnosis, Pearson correlation value of 0.41, p=0.009. In this small cohort of 42 patients, we were unable to demonstrate any correlation of LRG1 with clinical outcome.
Conclusion:
Plasma LRG1 protein showed a high sensitivity and high specificity in differentiating AML plasma from normal plasma. It also showed a strong correlation with total white cell count in AML patients. Further evaluation of LRG1 protein plasma level in AML patients in longitudinal manner is required to validate its utility in monitoring for minimal residual disease and early detection of leukemic relapse.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.