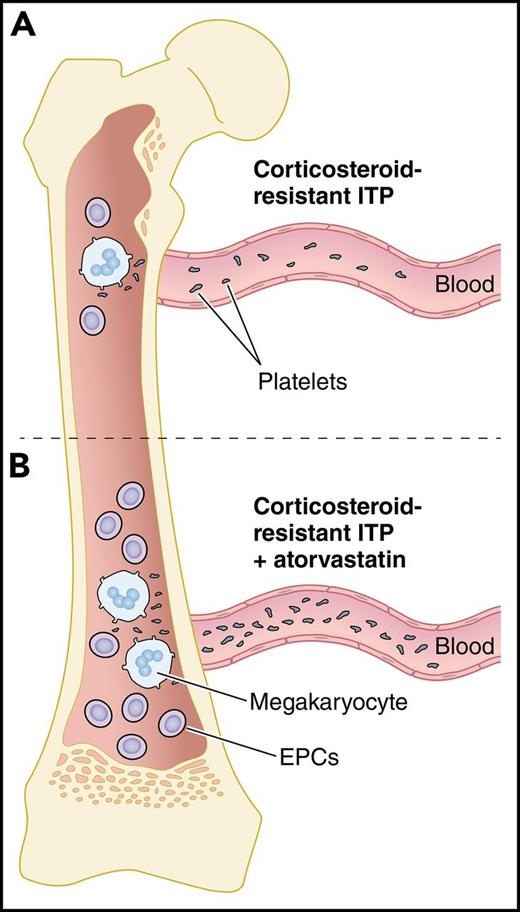
In this issue of Blood, Kong et al show that bone marrow (BM) endothelial progenitor cells (EPCs) in patients with corticosteroid-resistant immune thrombocytopenia (ITP) are reduced and dysfunctional but can be improved with atorvastatin, suggesting a potential novel therapy for ITP.1
Atorvastatin increases EPCs and thrombopoiesis in corticosteroid-resistant ITP. (A) In the BM of corticosteroid-resistant ITP, decreased numbers and dysfunctional EPCs lead to platelet underproduction. (B) Upon treatment of corticosteroid-resistant ITP with atorvastatin, the number and function of EPCs are improved by downregulating the p38 MAPK pathway and upregulating the Akt pathway, leading to maturation of megakaryocytes and increased platelet production. Professional illustration by Patrick Lane, ScEYEnce Studios.
Atorvastatin increases EPCs and thrombopoiesis in corticosteroid-resistant ITP. (A) In the BM of corticosteroid-resistant ITP, decreased numbers and dysfunctional EPCs lead to platelet underproduction. (B) Upon treatment of corticosteroid-resistant ITP with atorvastatin, the number and function of EPCs are improved by downregulating the p38 MAPK pathway and upregulating the Akt pathway, leading to maturation of megakaryocytes and increased platelet production. Professional illustration by Patrick Lane, ScEYEnce Studios.
Immune thrombocytopenia (ITP) is an acquired immune-mediated disorder characterized by a platelet count <100 × 109/L and an increased risk of bleeding. Historically, ITP was believed to be caused primarily by platelet autoantibodies, leading to accelerated platelet clearance by macrophages. More recently, studies have identified the role of platelet autoantibodies and cytotoxic T cells in impeding megakaryocyte development and maturation in the BM, resulting in decreased platelet production.2,3
The goal of treatment in ITP is to achieve a hemostatic platelet count while minimizing treatment-related morbidity. Typically, adults with ITP who require therapy are initially treated with corticosteroids, supplemented as needed with IV immunoglobulin or anti-D. Although most patients respond to first-line therapy, 10% to 20% do not and an additional ∼50% relapse when first-line therapy is tapered.4 In these individuals, splenectomy or second-line drugs such as rituximab, thrombopoietin-receptor agonists (TPO-RAs), and immunosuppressive agents may be used to maintain a hemostatic platelet count, often at significant cost and toxicity.5
Management decisions in ITP are dependent on multiple factors such as platelet count, bleeding symptoms, age, lifestyle, patient values and preferences, health-related quality of life, and side effects associated with therapy. Notably absent from this list is disease mechanism. Although the pathophysiology of ITP undoubtedly differs among patients, treatment decisions are currently made without regard for the disease mechanism(s) operating in an individual patient.
The pathogenesis of ITP is heterogeneous and the success of different treatments is likely to depend, in part, on the mechanism(s) leading to thrombocytopenia in different patients. Although platelet counts are low in ITP, the level of circulating thrombopoietin is not markedly increased as it is in some other thrombocytopenic disorders.6 This observation has led to development of the TPO-RAs, which produce platelet responses in up to 80% of patients with ITP by enhancing megakaryopoiesis and thrombopoiesis. The success of the TPO-RAs suggests that targeting other pathways involved in megakaryopoiesis may be a fruitful strategy for drug development in ITP.
Megakaryocytes in ITP patients show impaired maturation and signs of degradation due to defects in the megakaryocytes and their environment.7 Among the cells in the BM niche implicated in supporting megakaryopoiesis and thrombopoiesis are the BM EPCs, which are known to be reduced and defective in patients with prolonged thrombocytopenia after allogeneic hematopoietic stem cell transplantation (allo-HSCT).8 In the current study, Kong et al show that BM EPCs may be involved in the pathogenesis of corticosteroid-resistant ITP as well. They found that the number of BM EPCs was reduced in patients with corticosteroid-resistant compared with corticosteroid-sensitive ITP (0.02% ± 0.003% vs 0.12% ± 0.04%; P = .0001), even after 7 days of culturing (5.5 ± 0.9 vs 17 ± 2.6; P = .0001). Precultured BM EPCs from patients with corticosteroid-resistant ITP had higher levels of reactive oxygen species (1214 ± 122 vs 591.2 ± 45.4; P < .0001) and apoptosis (33.1% ± 3.6% vs 22.4% ± 4.3%; P = .02) and decreased functional characteristics such as migration (86.2 ± 5.6 vs 148.8 ± 14.8; P < .0001) and angiogenesis (3514 ± 437 vs 6268 ± 800.7; P = .02), all of which could contribute to impaired megakaryopoiesis (see figure panel A).
Treatments that induce platelet production by enhancing the BM microenvironment could be beneficial for corticosteroid-resistant ITP. Atorvastatin is a widely used drug for the treatment of primary hypercholesterolemia and mixed dyslipidemia by inhibiting 3-hydroxy-3methyl-glutaryl-coenzyme A reductase. Among its activities, it is known to improve the mobilization and function of EPCs. Recently, Shi et al showed the effectiveness of atorvastatin in increasing the functional characteristics of EPCs from patients with poor graft function after allo-HSCT.8 In the current study, Kong et al found that atorvastatin increased the total number of BM EPCs in corticosteroid-resistant ITP compared with untreated cells (2.3- ± 0.4-fold; P = .003). In addition, when atorvastatin was used, the function of BM EPCs from corticosteroid-resistant ITP was improved by downregulating the p38 MAPK pathway (0.8 ± 0.1-fold; P = .003) and upregulating the Akt pathway (2.5- ± 0.3-fold; P = .0002). These functional improvements led to increased megakaryocyte numbers (1.6- ± 0.2-fold; P = .02) and platelet release (2.6- ± 0.6-fold; P = .02) (see figure panel B). Similar effects were observed with other p38 inhibitors and N-acetyl-l-cysteine, a reactive oxygen species scavenger.
Their observations led Kong et al to hypothesize that atorvastatin would increase the platelet count in patients with corticosteroid-resistant ITP. In a pilot study, they treated 13 such patients, 9 of whom (69%) experienced a response. In these 9 individuals, the median platelet count rose from 21 × 109/L before treatment to 61 × 109/L on treatment (P = .004). The median time to response was 25 days. All of these individuals were on concomitant therapies (eg, prednisone, danazol), which could have contributed to the observed responses.
Although tantalizing, the results of this small pilot study require confirmation in a larger study, ideally a randomized placebo-controlled trial with careful restrictions on the use of concomitant therapy. The interesting findings of Kong et al suggest other avenues for future investigation as well. Immunomodulatory roles of statins have been identified in other autoimmune diseases such as multiple sclerosis and systemic lupus erythematosus.9 Future studies should identify whether atorvastatin has an immunomodulatory effect on resident immune cells in the BM of ITP patients, consequently improving the BM niche and facilitating clinical responses.
In summary, the findings of Kong et al show that impaired EPCs are present in patients with corticosteroid-resistant ITP and that their function is improved with atorvastatin (see figure). More broadly, they serve as a reminder that ITP is not one disease but rather a heterogeneous group of diseases with varying disease mechanisms. As the manifold mechanisms of ITP are unraveled, we will continue to move closer to the goal of a pathogenesis-oriented approach to therapy, an approach in which treatment is matched to patient, increasing response rates and decreasing the use of toxic yet ineffective options.10
Conflict-of-interest disclosure: The authors declare no competing financial interests.