Key Points
R41Q and R46Q point mutations in PAR1 in mice enabled studies of APC’s in vivo mechanism of action in lethal sepsis and ischemic stroke.
APC-biased, PAR1-dependent signaling due to cleavage at R46 in PAR1 is required for APC’s in vivo benefits in sepsis and ischemic stroke.
Abstract
Activated protein C (APC) cleaves protease-activated receptor 1 (PAR1) in vitro at R46 to initiate beneficial cell signaling; however, thrombin and APC can cleave at R41. To elucidate PAR1-dependent aspects of the pharmacologic in vivo mechanisms of APC, we generated C57BL/6 mouse strains carrying QQ41 or QQ46 point mutations in PAR1 (F2r gene). Using these strains, we determined whether or not recombinant murine signaling-selective APC mutants would reduce septic death or provide neuroprotection against ischemic stroke when mice carried PAR1-homozygous mutations that prevent cleavage at either R41 or R46. Intercrossing PAR1+/R46Q mice generated expected numbers of PAR1+/+, PAR1+/R46Q, and R46Q/R46Q offspring whereas intercrossing PAR1+/R41Q mice gave decreased R41Q/R41Q homozygotes (resembling intercrossing PAR1+/PAR1-knockout mice). QQ41-PAR1 and QQ46-PAR1 brain endothelial cells showed the predicted retention or loss of cellular responses to thrombin receptor-activating peptide, thrombin, or APC for each PAR1 mutation. In sepsis studies, exogenous APC reduced mortality from 50% to 10% in Escherichia coli–induced pneumonia for wild-type (Wt) PAR1 and QQ41-PAR1 mice (P < .01) but had no benefit for QQ46-PAR1 mice. In transient distal middle cerebral artery occlusion stroke studies, exogenous APC significantly reduced infarct size, edema, and neuronal apoptosis for Wt mice and QQ41-PAR1 mice but had no detectable benefits for mice carrying QQ46-PAR1. In functional studies of forelimb-asymmetry and foot-fault tests at 24 hours after stroke induction, signaling-selective APC was beneficial for Wt and QQ41-PAR1 mice but not QQ46-PAR1 mice. These results support the concept that APC-induced, PAR1-dependent biased signaling following R46 cleavage is central to the in vivo benefits of APC.
Introduction
The G-protein–coupled receptor (GPCR), protease-activated receptor 1 (PAR1),1 is activated not only by thrombin but also by activated protein C (APC), and these proteases cause diverse biologic activities.2-7 Other proteases also can cleave PAR1.8-11 PAR1-dependent signaling in endothelial cells initiated by thrombin leads to endothelial barrier weakening and proinflammatory effects whereas that initiated by APC leads to endothelial barrier stabilization and anti-inflammatory and antiapoptotic effects.5-7 Some insights into these opposing effects came from reports that PAR1 localization in caveolae is required for APC’s protective signaling.12,13 Nonetheless, how PAR1 activation could mediate contradictory biologic effects remained enigmatic until the concept of GPCR-biased signaling was firmly demonstrated for PAR1.14-16
APC and APC variants pharmacologically provide a remarkably diverse range of life-saving benefits in preclinical injury and disease models, and for APC’s benefits in many preclinical studies, PAR1 appears to be required.5-7 For example, the multiple neuroprotective activities of APC and the signaling-selective variant, 3K3A-APC, that led to a clinical trial of 3K3A-APC for ischemic stroke (RHAPSODY trial), require PAR1 and other receptors for neuroprotective benefits.4,5,7,17-21 Additionally, for reducing sepsis-induced death in mice, APC requires PAR1 based on studies using PAR1 gene knockout mice.22-24
Because the major mechanistic tool used for studies of mechanism of action for APC in vivo has been to compare PAR1-knockout mice to control mice, no clear details for in vivo mechanism of action of APC can be inferred; only the inference that PAR1 is required is validated from studies of knockout or knockdown vs wild-type (Wt) mice. An obvious confounding factor inherent to the use of knockout mice for studies of mechanism of multifunctional receptors such as PAR1 is that loss of opposing functional effects (eg, the deleterious effects of thrombin vs the beneficial effects of APC) inadvertently mask the receptor’s true contribution to unknown mechanisms of action, thereby falsely marginalizing the receptor’s important involvement. Another notable limitation of interpreting murine receptor gene knockout or knockdown studies in mechanistic terms arises because each GPCR, such as PAR1, is a member of an extensive protein-protein interaction module which, as 1 node in 1 or more networks, initiates signaling. Multimolecular assemblies involving scaffold proteins and receptors are critical for normal flows of cellular information.25 Therefore, knockout or knockdown of PAR1 will necessarily disrupt the PAR1 interactome, with potentially serious indirect effects on cell signaling that confound simple interpretations of in vivo studies using PAR1 knockouts or knockdowns. This confounding reality for interpreting receptor knockout studies is certainly relevant for PAR1 as it is known to interact with other PARs and other receptors as well as scaffold and adaptor proteins.26-34
To assess whether APC’s life-saving pharmacologic effects involve biased signaling via PAR1 due to cleavage at Arg46 and to circumvent the limitations of PAR1 gene knockouts, we constructed mice strains carrying the point mutations of R46Q or R41Q in PAR1 because the first mutation would ablate APC’s cleavage at R46 whereas the second mutation would prevent cleavage at R41 by APC or thrombin. To evaluate in vivo mechanisms, we used these mice strains and littermate control mice in 2 clinically relevant murine preclinical injury models, that is, sepsis (Escherichia coli–induced pneumonia) and ischemic stroke (middle cerebral artery [MCA] occlusion [MCAO] injury). The results presented here strongly support the simple concept that the mechanism of action for the in vivo pharmacologic benefits of APC requires biased signaling induced by APC’s noncanonical cleavage of PAR1 at R46.15
Materials and methods
Materials
Sources for materials were as follows: thrombin and plasma-derived APC (Enzyme Research Laboratories, South Blend, IN); mouse anti–extracellular signal-regulated kinase 1/2 (ERK1/2) (3A7), rabbit anti-pThr202/Tyr204-ERK1/2 (197G2), mouse anti-Akt (40D4), rabbit anti-pSer473Akt (D9E), mouse anti-Actin, and Draq5 dye (Cell Signaling Technology, San Diego CA); mouse anti-PAR1 (6A7H; Abbiotec, San Diego, CA); mouse anti–endothelial protein C receptor (RCR-16; Biolegend, San Diego, CA); infrared dye–conjugated secondary antibodies (LI-COR Biosciences, Lincoln, NE); bovine serum albumin (BSA; Calbiochem, EMD Millipore, Concord, MA); thrombin receptor activation peptide 10-mer (TRAP10; Anaspec, Fremont, CA); Dulbecco phosphate-buffered saline (PBS), penicillin streptomycin glutamine solution (Gibco, Carlsbad, CA); papain dissociation system (Worthington Biochemical Corp, Lakewood, NJ); heparin, Nutrient Mixture F-12 Ham medium, puromycin dihydrochloride, 22% BSA solution, ascorbic acid, collagen from calf skin, endothelial cell growth complements (Sigma-Aldrich Inc, St. Louis, MO); fetal bovine serum (FBS; Omega Scientific Inc, Tarzana, CA); Fluo-8 dye from AATBioquest (Sunnyvale, CA); and paraformaldehyde (Pierce Chemical), Thermo Fisher Scientific Inc. The transendothelial electrical resistance (TER) iCELLigence system and TER plates were purchased from ACEA Biosciences Inc (San Diego, CA).
PAR1 mutant mice and PAR1 genotyping
See supplemental Data (available on the Blood Web site) for details.
Brain endothelial cell isolation
Mouse brain endothelial cells (BECs) were isolated from 8- to 10-week-old mice.35 Briefly, tools were sterilized by flame for isolation of the brain. Mice were anesthetized by intraperitoneal (i.p.) injection of ketamine (90 mg/kg) and xylazine (10 mg/kg). Brains were removed surgically by dissecting through the dorsal side of the head and then immersed in minimal essential medium, and the tissue was diced by razor. Chopped tissue was collected in a 15-mL universal tube and centrifuged to wash out the medium. Furthermore, tissue was digested in 5 mL of medium containing papain (20 U/mL) and DNAse (2000 U/mL) at 37°C for 1 hour 10 minutes. Upon digestion, microvessels from the brain were broken up by homogenizing the tissue using 19-gauge and 21-gauge needles. The brain homogenate was treated with BSA solution (final 11% protein). The BSA solution containing brain homogenates was centrifuged to isolate the myelin and BSA solution layer and to collect the cell pellet. Thereafter, the pellet was washed, and the cells were grown on type I collagen-coated 6-well plates with complete BEC medium (F-12 Ham medium plus 10% FBS plus 30 µg/mL endothelial cell growth supplement [ECGM] plus 4 mM l-glutamine plus 40 µg/mL heparin plus 2.5 µg/mL ascorbate). After 12 hours, cell medium was replaced with 4 µg/mL puromycin dihydrochloride containing ECGM plus FBS-F12 Ham medium and grown for 2 days. Thereafter, the medium was changed to ECGM plus FBS-F12 Ham medium.
In-Cell Western
First-passage BECs were grown in black clear-bottom 96-well plates (Corning) until confluent. Cells were washed and starved overnight with F12 medium containing 0.1% BSA. Cells were treated with different concentrations of stimulating agent at 37°C in starvation medium (F12 medium containing 0.1% BSA) for 5 minutes (thrombin or TRAP10) or for 1 hour (APC). After a brief wash with ice-cold PBS, 0.15 M NaCl, 0.02 M phosphate, pH 7.4, cells were fixed in methanol free 4% paraformaldehyde for 20 minutes at room temperature and subsequently washed and permeabilized with 0.1% Triton X-100 in PBS for 15 minutes. Plates were then blocked with Odyssey blocking buffer supplemented with 5% BSA for 2 hours. Phospho-ERK or phospho-Akt was detected using the In-Cell Western module of Odyssey Imager with Image Studio software version 2.0. Fluorescence signals were corrected for cell number and background staining, and signals were normalized to buffer only controls at the corresponding time point.
Pneumonia sepsis in mice
All procedures were approved by the institutional animal care and use committee at The Scripps Research Institute in compliance with National Institutes of Health guidelines. An E coli strain K1-pneumonia sepsis protocol was adapted from Gupta el al.36 Mice were anesthetized by i.p. injection of ketamine (90 mg/kg) and xylazine (10 mg/kg). E coli (106 colony-forming units [CFUs] in 25 µL of PBS) were instilled intratracheally into anesthetized male mice. At both 15 minutes before receiving bacteria and at 6 hours afterward, anesthetized mice were given recombinant murine 5A-APC (0.1 mg/kg body weight) or PBS as a vehicle control in 100 µL IV in the eye. Mice were observed for death at regular intervals over 7 days after APC treatment.
Transient distal MCAO
All procedures were approved by the institutional animal care and use committee at the University of Southern California in compliance with National Institutes of Health guidelines. Male QQ41-PAR1 mice and QQ46-PAR1 mice and their littermate controls (25-35 g) were anesthetized with 100 mg/kg body weight i.p. ketamine and 10 mg/kg body weight xylazine. Rectal temperature was maintained between 36.5°C and 37.0°C using a feedback-controlled heating system. Transient distal MCAO was performed using a modified technique, as previously reported.20,37 In brief, under the surgical microscope, the left common carotid artery was isolated through a neck incision and ligated using a 5-0 silk suture. A skin incision was made between the left orbit and tragus. After the temporalis muscle was incised and retracted laterally to expose the squamous bone, a craniotomy (2 mm in diameter) was performed at the juncture of the zygoma and squamous bones to localize the left MCA. The MCA was ligated with a 9-0 nylon suture (S & T AG, Neuhausen, Switzerland) for 60 minutes. The right common carotid artery was transiently occluded for 20 minutes and then released. The MCA remained occluded for 60 minutes, at which time the blood flow to the MCA territory was restored by removing the suture. 3K3A-APC (0.04 mg/kg) or vehicle was administrated IV 4 hours after stroke. Forelimb-asymmetry and foot-fault testing38,39 were performed at 0 and 24 hours after MCAO. The mice were euthanized 24 hours after MCAO. The infarct volume was measured on coronal sections using cresyl-violet staining as described previously.40,41 Fluoro-Jade C staining was used to identify the MCAO-induced degenerating neurons as described.40 Extravascular fibrin deposition was investigated to show blood-brain barrier damage after MCAO.42
Embryo isolation
Mice were allowed to breed overnight and checked for a plug in the morning to assess successful mating. Mice with plugs were kept aside for embryo isolation at embryonic (E) stage days 10 (E10) and 12 (E12). Isolated embryos were photographed for visual development purposes, and tissues were taken for genotyping.
Calcium release assay
Intracellular calcium release by endoplasmic reticulum was measured by plate-based assays with the help of FLIPER tetra (Perkin Elmer). Fluo-8 dye was used according to the manufacturer’s protocol. BECs (5000 cells per well) were plated on 384 black clear-bottom plates and grown for 24 hours. Cells were starved overnight and then cell culture medium containing 5 µM Fluo-8 dye in 0.02% Pluronic F-127 was added. Plates were incubated for 30 minutes at 37°C and then 15 minutes at room temperature to acclimatize before addition of ligand. Ligands (TRAP10 and thrombin) were prepared in cell culture medium and added to cells in 384 well plates. Experiments were set up in the FLIPR tetra apparatus using 490 nm excitation and 514 nm emission. Data for relative fluorescence units (RFUs), which reflected intracellular calcium release, were analyzed by Screenworks version 3.1 (PerkinElmer).
TER assay
BECs were grown to confluence over gold microelectrode TER plates connected to an iCELLigence machine (ACEA Biosciences) as described.43 Cell index values were normalized prior to addition of thrombin, and results were expressed as a percentage of maximal barrier disruption observed for maximal thrombin concentration.
Statistical analysis
Significance was analyzed using 2-way analysis of variance (ANOVA) unless otherwise indicated in the figure legends. Survival in sepsis studies was analyzed by log rank.
Results
Novel C57BL/6 mice strains carrying Arg41Gln or Arg46Gln point mutations in PAR1 were generated
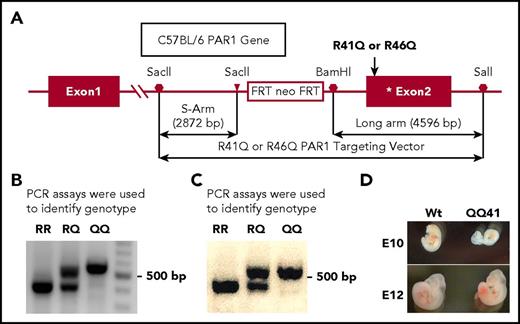
Murine gene knockout studies do not distinguish direct from indirect effects of the protein whose gene is knocked out. To enable studies that address the question of whether the specific amino acid where APC cleaves PAR1 for biased signaling, namely R46, is required for APCs in vivo benefits, we created C57BL/6 strains carrying either the R41Q or R46Q mutations in PAR1, as described in Figure 1A. Heterozygous mice were bred and genetic screening of offspring was done using polymerase chain reaction (PCR) (Figure 1B-C). PAR1 is vital for normal embryonic development as previously shown by breeding of heterozygous PAR1-knockout mice (PAR1+/PAR1-knockout) which exhibit partial embryonic loss, such that only around half of PAR1-deficient embryos survive.44,45 Genotype offspring data (Table 1) showed Mendelian frequency for QQ46-homozygous offspring produced by intercrossing of PAR1+/R46Q-PAR1 mice whereas non-Mendelian frequency was observed for the QQ41 homozygotes produced by intercrossing of PAR1+/R41Q-PAR1 mice, similar to that reported for intercrossing PAR1+/PAR1-knockout mice where homozygous-knockout mice yields were half the normal predicted amount.45 Because of this similar outcome for heterozygote mating, some observations were made at different embryonic developmental days. We observed partial embryonic loss around E10 stage and smaller embryos (eg, see photos in Figure 1D). These limited observations tend to imply that thrombin-induced PAR1 signaling due to cleavage at R41 is vital for normal embryonic development whereas cleavage at R46 is not vital for normal embryonic development. However, no systematic evaluation of embryonic development was undertaken for the intercrossing of PAR1+/R41Q-PAR1 mice, and a more focused study of embryogenesis is needed to draw firm conclusions about the effects of the R41Q-PAR1 point mutation on embryogenesis.
Novel C57BL/6 mice strains carrying either Arg41Gln or Arg46Gln mutations in PAR1 were generated. (A) C57BL/6 embryonic stem cells were used to generate mutations in mice using homologous recombination methods to incorporate the targeting vector pBS-FRT-PGK-NEO-FRT, as outlined in the panel and as described in supplemental Data. (B-C) To identify genotype for offspring from mating of QQ41 and QQ46 heterozygotes, PCR assays gave clear indication of genotypes, namely RR, RQ, or QQ, as shown for residues (B) 41 and (C) 46, respectively (see supplemental Data for methods). The DNA gel images were obtained from Bio-Rad GELDOC XR equipped with the image software Quantity One from Bio-Rad (Hercules, CA). (D) To record fetus development as observed at days 10 (E10) and 12 (E12) following plug formation, pictures of Wt and QQ41 embryos were taken under normal lighting. At E10, some QQ41 embryos were significantly smaller and underdeveloped compared with Wt embryos; however, no systematic analysis of embryogenesis was made.
Novel C57BL/6 mice strains carrying either Arg41Gln or Arg46Gln mutations in PAR1 were generated. (A) C57BL/6 embryonic stem cells were used to generate mutations in mice using homologous recombination methods to incorporate the targeting vector pBS-FRT-PGK-NEO-FRT, as outlined in the panel and as described in supplemental Data. (B-C) To identify genotype for offspring from mating of QQ41 and QQ46 heterozygotes, PCR assays gave clear indication of genotypes, namely RR, RQ, or QQ, as shown for residues (B) 41 and (C) 46, respectively (see supplemental Data for methods). The DNA gel images were obtained from Bio-Rad GELDOC XR equipped with the image software Quantity One from Bio-Rad (Hercules, CA). (D) To record fetus development as observed at days 10 (E10) and 12 (E12) following plug formation, pictures of Wt and QQ41 embryos were taken under normal lighting. At E10, some QQ41 embryos were significantly smaller and underdeveloped compared with Wt embryos; however, no systematic analysis of embryogenesis was made.
Genotype distribution of offspring from intercrossing of PAR1+/R41Q-PAR1 or of PAR1+/R46Q-PAR1 mice to establish embryonic survival of homozygous mutants
| PAR1 genotype distribution . | ||
|---|---|---|
| R41Q frequency, % (n = 162) . | Strain . | R46Q frequency, % (n = 198) . |
| 37 | Wt | 26 |
| 49 | Heterozygous | 47 |
| 14** | Homozygous | 27 |
| PAR1 genotype distribution . | ||
|---|---|---|
| R41Q frequency, % (n = 162) . | Strain . | R46Q frequency, % (n = 198) . |
| 37 | Wt | 26 |
| 49 | Heterozygous | 47 |
| 14** | Homozygous | 27 |
P < .01.
Arg41Gln and Arg46Gln PAR1 point mutations cause predicted cellular responses to thrombin, TRAP, and APC
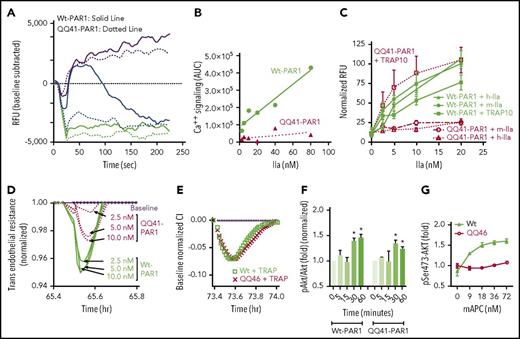
Multiple studies were performed to verify that the mutated PAR1 proteins in homozygote mice carrying either QQ41-PAR1 or QQ46-PAR1 genes were expressed and could exhibit predictable functional activities. Because ischemic stroke was one target indication, we used microvascular BECs isolated from brains of mutated mice. A hallmark of thrombin-mediated PAR1 signaling is rapid intracellular calcium release. Thrombin treatment (20 nM) of control BECs from Wt mice showed a normal response (Figure 2A solid blue line) whereas thrombin did not elicit any significant Ca++ release for BECs from QQ41-PAR1 mice (Figure 2A dashed blue line). Ionomycin gave the same normal predicted Ca++ flux response for Wt- and QQ41-PAR1 BECs (Figure 2A purple lines), thereby validating the assay. When the positive Ca++ flux was quantified at different thrombin doses as area under the curve for Ca++ flux, BECs from Wt, but not from QQ41-PAR1, mice showed a normal dose response (Figure 2B), indicating, as predicted, that the point mutation of R41 to Q ablated thrombin-induced Ca++ flux in BECs. To demonstrate the presence and function of QQ41-PAR1 in BECS from the mutated mice, a 10-mer PAR1 peptide, which is the TRAP10 produced by thrombin cleavage at R41, was used.15 As predicted for a normal level of PAR1 being present, human and murine thrombin elicited robust ion flux in Wt-PAR1 BECs but not in QQ41-PAR1 BECs (Figure 2C). However, the PAR1 agonist peptide, TRAP10, initiated Ca++ flux similarly in Wt-PAR1 and QQ41-PAR1 BECs, indicating the presence of normally functioning PAR1 receptors that are incapable of being activated by thrombin cleavage at Arg41 in the QQ41-PAR1 homozygous mutated mice (Figure 2C).
Arg41Gln and Arg46Gln PAR1 point mutations cause the predicted cellular responses to thrombin, TRAP, and APC as determined using microvascular BECs. (A) After BECs from QQ41-PAR1 mice or Wt mice were seeded in 384 well plates and the calcium dye Fluo-8 AM was added and incubated for 30 minutes, calcium release was triggered by addition of 20 nM thrombin (final concentration) (blue lines), ionomycin (purple lines), or control buffer (black lines), and calcium flux was recorded over 240 seconds. Solid lines represent results for BECs from Wt mice and dashed lines represent data for BECs from QQ41-PAR1–homozygous mice. (B) Area under curves (AUC) for calcium release (seen in panel A) were plotted for different doses of thrombin, which showed that thrombin gave the predicted dose-dependent increase in calcium ion release in Wt BECs whereas thrombin failed to initiate ion flux in BECs from QQ41-PAR1 mice. (C) Calcium flux was determined as described in “Materials and methods” following addition of different doses of a 10-mer TRAP10 and of mouse (m) or human (h) thrombin at varying doses to BECs from QQ41-PAR1 mice or Wt mice. Data points represent the normalized relative integrated total ion flux (RFU) increase that was recorded over 4 minutes compared with controls. Error bars represent standard error of the mean (SEM) for at least 3 separate experiments. Significance was analyzed using 2-way ANOVA. *P < .05. (D) Time-dependent changes in TER caused by thrombin (2.5, 5, and 10 nM) were recorded for BECs from Wt mice and QQ41-PAR1 mice. (E) The changes in TER caused by 20 μM TRAP10 for cultured BECs obtained from Wt mice and QQ-PAR1 mice were recorded. (F-G) Phosphorylation of Ser473 in Akt in BECs obtained from Wt mice and QQ41-PAR1 mice caused by murine APC (mAPC; 90 nM final in panel F) or BECs obtained from Wt mice and QQ46-PAR1 mice was determined. Total Akt antigen levels were used as loading controls to determine the ratio of phosphorylated Akt (pAkt)/Akt, which was normalized to 1.0 for no APC at zero time. Blots were scanned on LICOR and quantified error bars represent SEM for at least 3 separate experiments. Significance was analyzed using 2-way ANOVA. CI, confidence interval.
Arg41Gln and Arg46Gln PAR1 point mutations cause the predicted cellular responses to thrombin, TRAP, and APC as determined using microvascular BECs. (A) After BECs from QQ41-PAR1 mice or Wt mice were seeded in 384 well plates and the calcium dye Fluo-8 AM was added and incubated for 30 minutes, calcium release was triggered by addition of 20 nM thrombin (final concentration) (blue lines), ionomycin (purple lines), or control buffer (black lines), and calcium flux was recorded over 240 seconds. Solid lines represent results for BECs from Wt mice and dashed lines represent data for BECs from QQ41-PAR1–homozygous mice. (B) Area under curves (AUC) for calcium release (seen in panel A) were plotted for different doses of thrombin, which showed that thrombin gave the predicted dose-dependent increase in calcium ion release in Wt BECs whereas thrombin failed to initiate ion flux in BECs from QQ41-PAR1 mice. (C) Calcium flux was determined as described in “Materials and methods” following addition of different doses of a 10-mer TRAP10 and of mouse (m) or human (h) thrombin at varying doses to BECs from QQ41-PAR1 mice or Wt mice. Data points represent the normalized relative integrated total ion flux (RFU) increase that was recorded over 4 minutes compared with controls. Error bars represent standard error of the mean (SEM) for at least 3 separate experiments. Significance was analyzed using 2-way ANOVA. *P < .05. (D) Time-dependent changes in TER caused by thrombin (2.5, 5, and 10 nM) were recorded for BECs from Wt mice and QQ41-PAR1 mice. (E) The changes in TER caused by 20 μM TRAP10 for cultured BECs obtained from Wt mice and QQ-PAR1 mice were recorded. (F-G) Phosphorylation of Ser473 in Akt in BECs obtained from Wt mice and QQ41-PAR1 mice caused by murine APC (mAPC; 90 nM final in panel F) or BECs obtained from Wt mice and QQ46-PAR1 mice was determined. Total Akt antigen levels were used as loading controls to determine the ratio of phosphorylated Akt (pAkt)/Akt, which was normalized to 1.0 for no APC at zero time. Blots were scanned on LICOR and quantified error bars represent SEM for at least 3 separate experiments. Significance was analyzed using 2-way ANOVA. CI, confidence interval.
Disruption of endothelial barrier integrity, which can be monitored using TER, is another typical PAR1-mediated activity of thrombin. As predicted, thrombin treatment of Wt BECs induced disruption of the endothelial barrier, but the disruption was significantly less for BECs isolated from QQ41-PAR1–homozygous mice (Figure 2D); for example, the robust endothelial barrier disruption caused by only 2.5 nM thrombin for Wt-PAR1 BECs had a barely perceptible effect for QQ41-PAR1 BECs. The very modest effect of thrombin seen here likely reflects that action of thrombin on PAR4, which is present in endothelial cells and which is known to mediate a low level of response to thrombin.46,47
To characterize PAR1 presence and function in QQ46-PAR1 BECs, studies showed that the TRAP10-induced disruption of endothelial barrier integrity was the same for Wt BECs and for BECs from homozygous QQ46-PAR1 BECs (Figure 2E), verifying that functional PAR1 molecules were present in QQ46-PAR1 BECs.
The ability of APC to induce phosphorylation of Akt was compared for the various BECs as APC can induce PAR1-dependent phosphorylation of Ser473 as determined by western blotting (supplemental Figure 1A).15,48 APC induced its typical time-dependent pattern of Akt phosphorylation for Wt BECs and QQ41-PAR1 BECs mice (Figure 2F), whereas, in dose-response studies (Figure 2G), APC failed to induce phosphorylation of Akt in QQ46-PAR1 BECs. Overall, these data show that BECs homozygous for the QQ41-PAR1 mutation have PAR1 that is not responsive to thrombin but is responsive to the PAR1 agonist, TRAP10; moreover, BECs homozygous for the QQ46-PAR1 mutation have PAR1 that is not normally responsive to APC. When thrombin-induced phosphorylation of ERK was studied, phosphorylated ERK was seen in BECs from control Wt mice but was not seen for BECs from QQ41-PAR1 mice (supplemental Figure 1B). Thus, BECs from 41QQ-PAR1–mutated mice have a predicted normal PAR1 function for APC-induced Akt phosphorylation due to cleavage at R46 and a predicted loss of thrombin-induced ERK phosphorylation due to cleavage at R41.
Arg46 in PAR1 is required for APC mortality reduction in bacterial sepsis
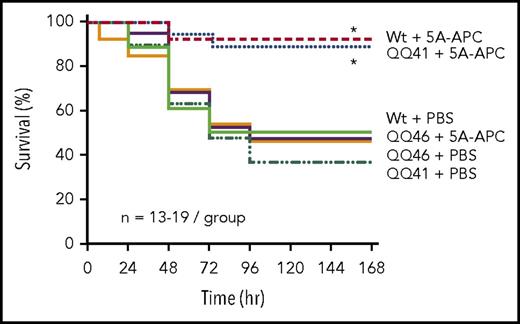
APC is therapeutic and reduces mortality in sepsis caused by lipopolysaccharide22 and bacteria.49,50 The requirement for R46 in PAR1 for APC’s ability to reduce death in murine sepsis was assessed in a bacterial E coli pneumonia sepsis model adapted from Gupta et al.36 Sepsis was induced by K-1 E coli instillation into trachea of mice at a dose sufficient to provoke 50% to 60% lethality in Wt mice or PAR1-knockout mice. Then, as expected, treatment with recombinant murine signaling-selective 5A-APC reduced death to ≤10% for Wt mice but not for PAR1-knockout mice (supplemental Figure 2). When sepsis was similarly induced in mice homozygous for QQ41 or QQ46, treatment of Wt mice and QQ41-PAR1 mice with 5A-APC reduced death to ≤10% whereas 5A-APC gave no significant reduction in death for QQ46-PAR1 mice (Figure 3). Thus, R46 in PAR1 is required for mortality reduction by APC in severe bacterial sepsis.
APC-mediated mortality reduction in bacterial sepsis is seen for QQ41-PAR1 mice but not for QQ46-PAR1 mice. The prosurvival effect of 5A-APC in E coli–induced pneumonia sepsis was tested in Wt and in PAR1 genetically modified mice. Sepsis was induced by instillation of 106 CFU E coli intratracheally. 5A-APC IV treatment was given at 15 minutes before E coli instillation and at 6 hours after infection. QQ41-PAR1 mice, QQ46-PAR1 mice, and Wt littermate controls were observed for survival following E coli infection and 5A-APC treatment over 7 days. Significance was analyzed using log rank. *P < .01.
APC-mediated mortality reduction in bacterial sepsis is seen for QQ41-PAR1 mice but not for QQ46-PAR1 mice. The prosurvival effect of 5A-APC in E coli–induced pneumonia sepsis was tested in Wt and in PAR1 genetically modified mice. Sepsis was induced by instillation of 106 CFU E coli intratracheally. 5A-APC IV treatment was given at 15 minutes before E coli instillation and at 6 hours after infection. QQ41-PAR1 mice, QQ46-PAR1 mice, and Wt littermate controls were observed for survival following E coli infection and 5A-APC treatment over 7 days. Significance was analyzed using log rank. *P < .01.
Arg46 in PAR1 is required for APC neuroprotection in ischemic stroke
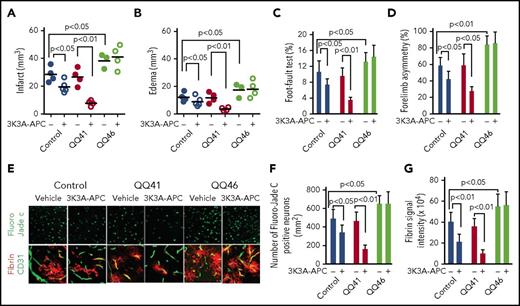
Wt-APC and signaling-selective APC mutants are neuroprotective in many studies.4,5,7,17-20 The requirement for R46 in PAR1 for the neuroprotective activities of APC in an ischemic stroke model was assessed. Treatment with murine recombinant signaling-selective 3K3A-APC after MCAO was neuroprotective in Wt mice and QQ41-PAR1 mice but not in QQ46-PAR1 mice based on many parameters (Figure 4). This conclusion is supported by data obtained at 24 hours after MCAO for infarct volume (Figure 4A), edema (Figure 4B), foot-fault test (Figure 4C), and forelimb-asymmetry test (Figure 4D) as well as for data for degenerating neurons (Figure 4E-F) and for fibrin deposition determined by anti-fibrin antibodies (Figure 4E,G). Based on these extensive data in this MCAO model, R46 in PAR1 is required for APC neuroprotection in ischemic stroke.
APC-mediated neuroprotection in ischemic stroke is observed in QQ41-PAR1 mice but not in QQ46-PAR1 mice. Data (mean values) for 24 hours after MCAO are shown for infarct volume (A), edema (B), foot-fault test (C), and forelimb-asymmetry test (D) for homozygous QQ41-PAR1 mice, homozygous QQ46-PAR1 mice, and the corresponding Wt controls treated with murine recombinant 3K3A-APC (0.04 mg/kg given IV 4 hours after MCAO) or vehicle. Bars indicate mean ± standard deviation (SD), n = 4-6 mice per group. Data for 24 hours after MCAO are seen for degenerating neurons determined by Fluoro-Jade C stain (E-F) and for fibrin deposition determined by anti-fibrin antibodies (E,G) for each mouse group (panel E, original magnification ×20). Treatment of mice with 3K3A-APC or vehicle is indicated by plus or minus signs under panels A-D, F, and G. Student t test and 1- or 2-way ANOVA followed by a post hoc Tukey test were used to determine statistically significant differences. The curves of functional recovery after stroke were compared using repeated-measures ANOVA. P < .05 was considered statistically significant.
APC-mediated neuroprotection in ischemic stroke is observed in QQ41-PAR1 mice but not in QQ46-PAR1 mice. Data (mean values) for 24 hours after MCAO are shown for infarct volume (A), edema (B), foot-fault test (C), and forelimb-asymmetry test (D) for homozygous QQ41-PAR1 mice, homozygous QQ46-PAR1 mice, and the corresponding Wt controls treated with murine recombinant 3K3A-APC (0.04 mg/kg given IV 4 hours after MCAO) or vehicle. Bars indicate mean ± standard deviation (SD), n = 4-6 mice per group. Data for 24 hours after MCAO are seen for degenerating neurons determined by Fluoro-Jade C stain (E-F) and for fibrin deposition determined by anti-fibrin antibodies (E,G) for each mouse group (panel E, original magnification ×20). Treatment of mice with 3K3A-APC or vehicle is indicated by plus or minus signs under panels A-D, F, and G. Student t test and 1- or 2-way ANOVA followed by a post hoc Tukey test were used to determine statistically significant differences. The curves of functional recovery after stroke were compared using repeated-measures ANOVA. P < .05 was considered statistically significant.
Interestingly, the QQ46 mutant had a statistically significant increase in infarct volume compared with WT mice, which may reflect the loss of endogenous APC neuroprotective signaling through PAR1. APC appeared to be more protective in the QQ41-mutant mice; one may speculate that this may be due to a loss of pathologic thrombin-PAR1 signaling.
Discussion
Like other GPCRs, PAR1 is capable of biased signaling.6,7,9-16 Although this has been well demonstrated with in vitro studies, there is a dearth of data relating to PAR1 in vivo biased signaling. One in vivo study showed that a 20-mer PAR1 peptide that begins with N47, which represents the new N-terminal PAR1 sequence following cleavage at R46, reduces vascular leakage, as does APC.15 To enable novel in vivo studies related to PAR1, mice carrying the point mutations R41Q or R46Q in PAR1 were created and used to assess requirements for R41 or R46 for APC’s benefits in sepsis and ischemic stroke. The striking findings here are that R46 is indeed required for APC’s pharmacologic benefits. In the conditions used for a lethal E coli–pneumonia sepsis model, APC reduced death from 50% to ∼5% for Wt and QQ41-PAR1 mice. However, when R46 was mutated to Q, APC lost its ability to reduce septic death in the mice carrying a PAR1 that could not be cleaved at R46 by APC. In a MCAO murine model for ischemic stroke in which APC demonstrates remarkable benefits, as seen previously and here,17-20 the mutation of R46 to Q ablated the neuroprotective benefits of APC as judged by multiple parameters including inter alia, infarct volume, animal behavior, and neuronal apoptosis. Thus, R46 in PAR1 is required for multiple in vivo pharmacologic benefits of APC. Because extensive in vitro studies15 implied that cleavage at R46 triggers APC-initiated biased signaling, these new findings strongly support the hypothesis that APC-initiated PAR1 biased signaling is an essential mechanistic component in vivo for the pharmacologic benefits of APC in these models.
Genotyping of offspring from mating of heterozygous QQ41-PAR1 or QQ46-PAR1 mice indicated that the former but not the latter mutation caused defective embryogenesis similar to that observed for the mating of heterozygous PAR1-knockout mice where significantly lower than predicted percentages of homozygous mice were found and where some embryos were smaller than normal.44,45 Additional studies showed that PAR1 knockout specifically in endothelial cells caused the decreased embryonic survival seen for PAR1 knockouts.51 Breeding of heterozygous mice carrying prothrombin mutations that prevent generation of α-thrombin while allowing generation of meizothrombin gave a similar pattern of embryonic survival, that is, half the expected homozygotes.52 Those studies generated the hypothesis that normal murine embryogenesis requires PAR1-dependent signaling initiated by α-thrombin due to cleavage at the canonical R41. Our findings strongly support this hypothesis because simply mutating 1 residue, namely R41, results in a similar breeding pattern as seen for PAR1-knockout heterozygotes44,45,51 or meizothrombin heterozygotes.52
The observation that R46 in PAR1 is required for the benefits of APC in the sepsis and stroke models used here does not rule out the likelihood that other signaling receptors also mediate some aspects of APC-initiated protective signaling in 1 or the other injury models. PAR3,4,53-57 Mac-1,58 apoER2,48,59 and Tie260,61 have been implicated as significant mediators for APC-initiated signaling.4,48,53-61 So, it may be that PAR1 biased signaling consequent to R46 cleavage is necessary but not sufficient for the optimal effects of pharmacologic APC. Further studies are needed to clarify such issues.
Genetically altered mice are invaluable for proof-of-concept mechanistic studies for how drugs achieve their desired effects or their off-target effects. The introduction of point mutations in PARs in mice for in vivo studies, as done here, enables insights not possible with gene knockout studies. For future studies of in vivo mechanism of action of various proteases, it would seem attractive to use mice or other species carrying point mutations in PAR genes. Much remains to be discovered about the structural basis for how the PARs accomplish their various modes of biased signaling. One study used a mouse strain carrying the point mutation of R37Q in PAR2 for mechanistic insights unrelated to biased signaling.50 The core structure of human PAR1 complexed to a high-affinity antagonist, vorapaxar, is known.62 However, nothing about the binding to PAR1 of its diverse N-terminal tethered ligands, which are generated by cleavage by thrombin or APC or other proteases, is known. Because PAR1-dependent biased signaling in vivo is strongly supported by our findings, more structural knowledge about how PAR1 interacts with its diverse N-terminal sequences will have very relevant biologic and pharmacologic implications.
PAR1 is cleaved not only by thrombin and APC but also by other proteases, for example, matrix metalloproteinases, elastase, plasmin, etc.8-11 Either the R41Q or the R46Q mutation might directly or indirectly influence the rates or specificity of cleavages by these other proteases due to the influence of altered subsites involved in substrate recognition by these proteases. Our 2 new mouse strains, or similar strains containing PAR1 point mutations, may help to give new insights for how the other proteases promote their signaling actions.
In summary, these studies strongly support the hypothesis that the in vivo mechanism of action for APC’s pharmacologic benefits for bacterial sepsis and for ischemic stroke involve biased signaling by PAR1 due to cleavage at R46 by APC. Moreover, these studies demonstrate the unique utility of mouse strains carrying the point mutations of R41Q or R46Q in PAR1 for deriving insights regarding a protease’s in vivo mechanism of action.
Presented in abstract and oral form at the 58th annual meeting of the American Society of Hematology, San Diego, CA, 3-6 December 2016. The abstract was awarded the Mary Rodes Gibson prize for the highest-scored trainee abstract in the hemostasis and thrombosis category.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health, National Heart, Lung, and Blood Institute grants HL104165 and HL130678 (L.O.M.), National Institute of Neurological Disorders and Stroke grant NS090904 (B.V.Z.), and National Heart, Lung, and Blood Institute grants HL031950, HL052246, and HL133728 (J.H.G.), and by the Fondation Suisse pour les Bourses en Médecine et Biologie (L.B.).
Authorship
Contribution: R.K.S., Y.W., Z.Z., N.G., J.A.F., X.X., L.B., G.M., S.K., and L.O.M. performed experiments; R.K.S., N.G., L.O.M., B.V.Z., and J.H.G. designed the study; R.K.S., Y.W., B.V.Z., and J.H.G. wrote the paper; and all authors read and approved the paper.
Conflict-of-interest disclosure: B.V.Z. is a founder of ZZ Biotech LLC, a biotechnology company with a mission to develop APC and its functional mutants for the treatment of stroke and other neurological disorders. L.O.M. and J.H.G. are inventors for some uses of APC mutants. J.H.G. is a consultant for ZZ Biotech LLC. The remaining authors declare no competing financial interests.
The current affiliation for R.K.S. is NantKwest, Inc, San Diego, CA.
The current affiliation for L.B. is Novo Nordisk, Copenhagen, Denmark.
Correspondence: John H. Griffin, Department of Molecular Medicine, The Scripps Research Institute, 10550 N. Torrey Pines Rd, La Jolla, CA 92037; e-mail: jgriffin@scripps.edu.
REFERENCES
Author notes
R.K.S. and Y.W. contributed equally to this study.
B.V.Z. and J.H.G. share senior authorship.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal