In this issue of Blood, Sinha et al describe the generation of 2 mouse lines with distinct point mutations in protease-activated receptor 1 (PAR1) that prevent the cleavage of the receptor by either thrombin or activated protein C (APC).1
Grand opening for PAR1 research in vivo. Professional illustration by Somersault18:24.
Grand opening for PAR1 research in vivo. Professional illustration by Somersault18:24.
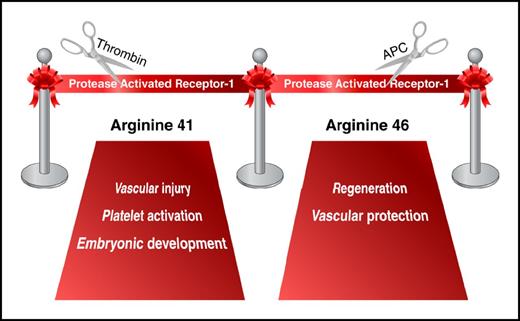
In addition to fibrinogen cleavage, the coagulation protease thrombin also induces intracellular signaling in platelets, endothelial cells, and other cell types. These cellular responses are mediated, in part, by activation of the G-protein-coupled receptor known as PAR1. The receptor is activated via a unique mechanism. Thrombin cleaves its N-terminal exodomain at the Arg41 residue, resulting in the generation of a new N-terminus that acts as a tethered ligand. The new N-terminus binds to the extracellular domain of the receptor and induces transmembrane signaling.2 Discovery of PAR1 and subsequent generation of PAR1-deficient mice have been crucial to the understanding of the role of thrombin in hemostasis, thrombosis, embryonic development, and many aspects of vascular inflammation.3
The protein C pathway is one of the endogenous anticoagulant systems. Protein C is converted to APC by the thrombin-thrombomodulin-endothelial protein C receptor complex on endothelial surfaces.4 In addition to its well-documented anticoagulant activity, APC and the cofactors of the protein C pathway exert cytoprotective effects by inhibiting cell apoptosis and attenuating inflammatory responses.4 Riewald and colleagues demonstrated that some of the beneficial effects of APC are mediated by PAR1 activation,5 raising the question of how PAR1 activation could lead to both pro- and anti-inflammatory responses. This paradox was expanded further by studies investigating the role of PAR1 in vascular permeability, in which thrombin-mediated PAR1 activation led to the disruption of endothelial barrier function, whereas the cleavage of PAR1 by APC prevented vascular leakage.4 Over time, more data have been generated to support both sides of the story, resulting in some polarization within the scientific community. It was even proposed that PAR1 activation is unlikely to play any role in the physiological or pharmacological effects of APC because the affinity of thrombin for PAR1 was about 10 000-fold greater and would simply outcompete APC for PAR1 cleavage.6
Clarification of this controversy came with the elucidation of G-protein–coupled receptor-biased signaling: 1 receptor, 2 different ligands, 2 different responses.7 In 2012, Mosnier and colleagues demonstrated that APC cleaves PAR1 sequences not only at Arg41 (the thrombin cleavage site) but also at Arg46.8 Data from in vitro experiments suggested that the PAR1 N-terminus generated by APC cleavage at Arg46 is a novel biased agonist that mediates cytoprotective PAR1 signaling.8 Two mouse lines generated by Sinha and colleagues help to further probe biased PAR1 signaling. Introduction of a point mutation in the PAR1 gene at Arg41 reproduced the partial embryonic lethality observed in PAR1-deficient animals but did not affect the APC-mediated protection in mouse models of sepsis and brain ischemia/reperfusion injury. In contrast, a mutation at Arg46 did not influence embryonic development; however, it rendered mice unresponsive to APC treatment, thereby demonstrating the requirement for cleavage at Arg46 for the beneficial effects of APC in vivo (see figure).
Mouse lines with point mutations affecting cleavage of the receptor by various proteases will be valuable tools to study in vivo PAR1 responses for which the PAR1 knockout mouse model was not optimal. These are useful tools to study not only the opposing functions of PAR1 but also the consequences of signaling mediated by various homo- and heterodimers formed by PAR1 and other members of the PAR family. In addition, mice with mutations in the PAR2 gene, which make the PAR2 receptor resistant to cleavage by factor Xa (PAR2 G37I) or completely insensitive to cleavage (PAR2 R38E), have recently been developed and will provide insight into candidate coagulation protease activation of PAR2 in various pathological conditions.9
Finally, thrombin-PAR1 signaling plays an important role in human platelet biology. However, in contrast to human platelets that express PAR1 and PAR4, mouse platelets express PAR3 and PAR4.3 This difference in PAR expression has limited the utility of mouse models in investigating the role of PAR1 in platelets. After several unsuccessful attempts, an overexpression of functional human PAR1 in mouse platelets has been recently reported,10 overcoming the remaining obstacle to studying PAR1 responses in vivo in mice. Armed with all the novel mouse models mentioned above, as well as with recently generated PAR1 and PAR2 floxed mice, we are entering a new, exciting era for PAR research.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal