Abstract
Platelets have long been recognized as key players in hemostasis and thrombosis; however, growing evidence suggests that they are also significantly involved in cancer, the second leading cause of mortality worldwide. Preclinical and clinical studies showed that tumorigenesis and metastasis can be promoted by platelets through a wide variety of crosstalk between platelets and cancer cells. For example, cancer changes platelet behavior by directly inducing tumor-platelet aggregates, triggering platelet granule and extracellular vesicle release, altering platelet phenotype and platelet RNA profiles, and enhancing thrombopoiesis. Reciprocally, platelets reinforce tumor growth with proliferation signals, antiapoptotic effect, and angiogenic factors. Platelets also activate tumor invasion and sustain metastasis via inducing an invasive epithelial-mesenchymal transition phenotype of tumor cells, promoting tumor survival in circulation, tumor arrest at the endothelium, and extravasation. Furthermore, platelets assist tumors in evading immune destruction. Hence, cancer cells and platelets maintain a complex, bidirectional communication. Recently, aspirin (acetylsalicylic acid) has been recognized as a promising cancer-preventive agent. It is recommended at daily low dose by the US Preventive Services Task Force for primary prevention of colorectal cancer. The exact mechanisms of action of aspirin in chemoprevention are not very clear, but evidence has emerged that suggests a platelet-mediated effect. In this article, we will introduce how cancer changes platelets to be more cancer-friendly and highlight advances in the modes of action for aspirin in cancer prevention. We also discuss the opportunities, challenges, and opposing viewpoints on applying aspirin and other antiplatelet agents for cancer prevention and treatment.
Introduction
Despite considerable progress in developing new approaches for cancer treatment over the past 2 decades, cancer continues to be an enormous challenge for public health. It is the second leading cause of mortality worldwide, and has overtaken cardiovascular diseases (CVDs) as the principal cause of death in United States.1,2 Aspirin, a widely used antiplatelet and anti-inflammatory agent, has emerged as perhaps the most promising drug for cancer prevention.3-5 Platelets are small anucleate blood cells generated from megakaryocytes in the bone marrow and also likely the lung.6,7 Since the late 1960s, scientists and clinicians have begun to notice the links between platelets and cancer.8 It has now become clearer that cancer cells can induce abnormalities in platelet number and function. In turn, platelets can promote tumor growth and metastasis.6,9-15
Overview of platelet functions
Platelets are key players in hemostasis and thrombosis, including those in tumor vasculature.16-18 At sites of vascular injury, platelet adhesion, activation and aggregation, and elaboration of procoagulant surface activity,19 are critical events to stop bleeding.20-22 Low platelet counts in blood, such as immune-mediated and chemotherapy-induced thrombocytopenias,23-27 may cause life-threatening bleeding.28-30 However, improper platelet activation and aggregation may result in thrombosis, leading to CVD.16,31-33 Importantly, 10% to 15% of cancer patients develop a cancer-associated thrombosis, especially venous thromboembolism, which is the second leading cause of death in cancer patients.10,34 Tumor-activated platelets likely contribute to these thrombotic events.35-38
Numerous studies have investigated the molecular basis in mediating thrombosis.16,39-41 Although fibrinogen has been documented to be required for platelet aggregation, recent evidence demonstrated that fibrinogen-independent platelet aggregation occurs in both animals42-45 and humans.46-48 Although proteins supporting this novel aggregation pathway remain to be studied, platelet αIIbβ3 integrin is essential,44 and several αIIbβ3 ligands such as fibronectin, thrombospondin-1, and counterreceptor cadherin 6 may be involved.21,39,49-52 These platelet receptors and their ligands also likely contribute to platelet–tumor interactions, metastasis, and cancer-associated thrombosis.
Reciprocal crosstalk between cancer and platelets
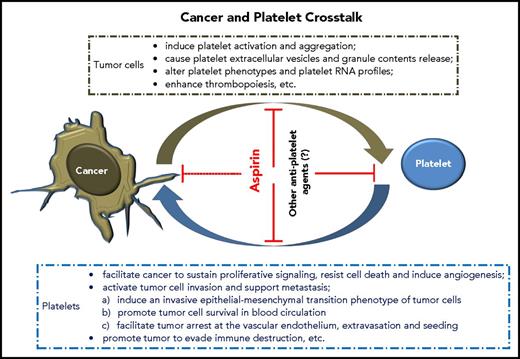
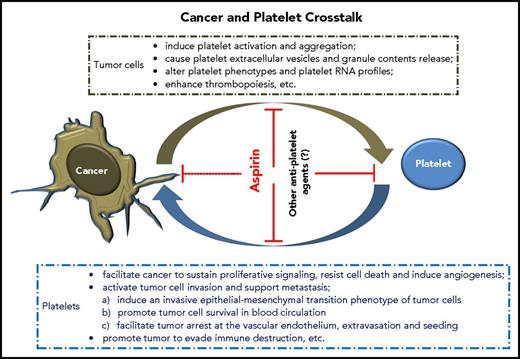
Elegant reviews have summarized the hallmarks of tumor cells acquired during their development, which control the transformation of normal cells to cancer (supplemental Data, available on the Blood Web site).61,62 Notably, cancer can “dictate” platelets to support these key processes. We now know that tumor cells and platelets maintain a complex, bidirectional interaction in the blood and tumor microenvironment (TME) (Figure 1), although further investigation of details related to these interactions is required.
How cancer changes platelets
TCIPA and formation of tumor-platelet aggregates
The concept of tumor cell–induced platelet aggregation (TCIPA) can be traced back to the first observation in the late 19th century.67 Despite the incompletely understood mechanism, which may vary depending on tumor type, platelet agonists (eg, thrombin, adenosine 5′-diphosphate) generated by the tumor cells and microenvironment seem to be the stimulators,68 followed by the interactions of various platelet receptors and ligands. It is currently unknown how many of these receptors (supplemental Data)52,69-78 are involved in TCIPA (ie, platelet–platelet, platelet–tumor, tumor–platelet–leukocyte aggregation11,12 ) and how they contribute to this process.
Several platelet receptors and their ligands, however, have been recently elucidated in TCIPA (Table 1). Platelet αIIbβ3, through binding fibrin(ogen) or fibrin–fibronectin complexes,50,51 bridges tumor αVβ3.79-81 Fibrin can be generated by the tumor cell tissue factor–initiated coagulation pathway. Platelet α6β1, through binding ADAM9 on tumor cells, enhances platelet activation and tumor cell extravasation.82 Binding of platelet P-selectin to tumor P-selectin ligands83-85 also mediates platelet–tumor cell microthrombi.86-88 Platelet Toll-like receptor (TLR) 4 promotes TCIPA and metastasis through interaction with tumor-released high-mobility group box 1 protein.89 In addition, platelet CLEC-2 induces TCIPA and thrombosis in tumor vessels and facilitates metastasis via ligation with tumor podoplanin.71,72 High podoplanin expression on brain tumors was correlated with increased platelet aggregation and risk of venous thromboembolism in patients.90
Studies on GPIb-IX-V complex, however, have inconsistent findings. GPIbα knockout mice showed reduced lung metastasis, indicating its supportive roles.91 Notably, de novo expression of von Willebrand factor (VWF) was also found in cancer cells of nonendothelial origin.92 Thus, platelet GPIbα likely binds to tumor VWF and mediates TCIPA and metastasis. It will be worthwhile to test whether Anfibatide, a new anti-GPIbα polypeptide isolated from snake venom, could reduce metastasis.93 Interestingly, a study also reported that a monoclonal antibody against GPIbα promoted melanoma metastasis.94 One cannot exclude that some anti-GPIbα antibodies may activate platelets,95-98 enhance TCIPA, and facilitate the observed metastasis.94 It is necessary to investigate whether these confounding effects resulted from the use of different animal models and are reproducible in other tumor cell lines by different anti-GPIbα antibodies. This information is important for the further development of antiplatelet drugs targeting GPIbα99 to control CVD and cancer.
Altogether, these studies demonstrate that tumor cells can activate platelets and induce TCIPA. There is no doubt that more platelet receptors and ligands will be identified in this process. Although TCIPA is not easily detected as a biomarker for cancer diagnosis and prognosis because of its relatively low frequency in peripheral blood, targeting these platelet receptor ligands may have great potential for new adjuvant antitumor therapies (Table 1).
Tumor cells induce platelet extracellular vesicle generation, granule release, and phenotype changes
Following activation, aggregation with tumor cells and exposure to shear stress, platelets release extracellular vesicles (EVs), such as exosomes and microparticles.100 Aggressive tumors are correlated with higher levels of platelet microparticles.101,102 It has been shown that microRNA-223 delivered by platelet-derived microparticles is significantly increased in patients with non–small cell lung cancer (NSCLC).103 Tumors also induce platelet granule release104 and phenotype changes in cancer patients by increasing the secretion of pro-angiogenic proteins (see “Platelets facilitate cancer to sustain proliferative signaling, resist cell death, and induce tumor angiogenesis”), such as vascular endothelial growth factor (VEGF).105 These cancer-associated features may be developed as early biomarkers for cancer screening.
Tumor cells alter platelet RNA profiles
It has recently been highlighted that tumors can also alter platelet RNA profiles.63,65,106-108 The exact mechanisms of RNA signature in TEP are not well understood. One mechanism might be via cancer cells releasing RNA into their local environment, likely through EV such as tumor-derived exosomes,109-111 and transferring mutant RNA into platelets.63,65,107,108 Indeed, platelets from cancer patients contained tumor-associated RNA biomarkers, such as EGFRvIII and PCA3 for glioma and prostate cancer, respectively.107 Although it is not clear how tumor-derived exosome uptake by platelets occurs, plasma membrane fusion, clathrin-mediated endocytosis, and phagocytosis may be involved.109 Importantly, messenger RNA (mRNA) sequencing of TEP can identify cancer patients with 96% accuracy and distinguish 6 primary tumor types, including NSCLC, glioblastoma, colorectal, pancreatic, hepatobiliary, and breast cancers with 71% accuracy.63 In addition, TEP accurately detected both early- and late-stage NSCLC.65 Because platelets are also anucleate cells and are easily isolated, this TEP-based RNA biosource, despite requiring further characterization, may serve as an attractive platform for liquid biopsy, which is a primarily blood-based, minimally invasive assay for cancer diagnosis, prognosis, and treatment monitoring in the context of precision medicine.112
Tumor cells enhance thrombopoiesis
The extent of thrombocytosis has a close relationship with the poor clinical outcome for the majority of malignancies, such as cancers of ovary, bladder, kidney, pancreas, esophagogastric, uterus, and, in particular, colorectal and lung.113,114 Thrombocytosis in primary care is also positively correlated with an increased risk of certain cancers.115 Evidence has shown that the increased thrombopoietic cytokine production by tumor and host tissues, such as interleukin-1 (IL-1), IL-3, IL-11, and particularly tumor-derived IL-6, is the predominant cause of hepatic thrombopoietin generation and thrombocytosis.116,117 Tumor-derived platelet factor 4 (PF4) has also been reported to promote platelet production.118 Intriguingly, we recently found that platelet GPIbα is required for platelet-induced hepatic thrombopoietin generation in humans and mice.119 It is currently unknown whether these tumor-released cytokines and platelet GPIbα can synergistically trigger thrombopoietin production. Therefore, thrombocytosis may be a cost-effective (ie, platelet count is an easy and inexpensive assay) and noninvasive biomarker for early cancer detection and poor prognosis.113-117
How platelets support tumor growth and metastasis
Platelets facilitate cancer to sustain proliferative signaling, resist cell death, and induce tumor angiogenesis
Recent evidence suggests that platelets have a direct effect on cancer cell proliferation. Platelet transforming growth factor β (TGF-β) increased the proliferation of ovarian cancer cells.121,122 Platelet microparticles also stimulated mitogen-activated protein kinases in lung carcinoma cells and increased cell proliferation.123 Interestingly, patients with clear cell renal cell carcinoma have remarkably increased platelet isoform of phosphofructokinase (PFKP), a rate-controlling enzyme of the glycolytic pathway. Suppression of PFKP decreased glycolysis in clear cell renal cell carcinoma cells, impaired cell proliferation, and induced apoptosis124,125 ; it is unknown whether platelets could transfer their PFKP mRNA to cancer cells. In addition, platelets and platelet lysates could cause mitochondrial uncoupling and resistance to apoptosis in leukemia cells.126 Collectively, these studies provide insights as to why patients with thrombocytosis usually have poor survival and enhanced resistance to chemotherapy. Indeed, experimental evidence shows that platelet depletion markedly reduced tumor weight and enhanced the efficacy of chemotherapy; conversely, platelet transfusion increased tumor size and decreased drug efficacy.27,127 Thrombocytopenia-induced tumor hemorrhage may also improve drug delivery.18 This raises a question whether we should increase the threshold for platelet transfusion in cancer patients with chemotherapy-induced thrombocytopenia.27,128
Platelets contain numerous proangiogenic factors, such as VEGF, platelet-derived growth factor (PDGF), basic fibroblast growth factor, and insulin-like growth factors.15,104,129-132 These proangiogenic factors induce formation of tumor-infiltrating blood vessels and may promote proliferation/differentiation of cancer-associated pericytes and fibroblasts in the TME.104,120 Platelets also contain antiangiogenic proteins, such as angiostatin, endostatin, thrombospondin-1, and PF4.133,134 In the TME, cancer cells may use platelets to predominate the angiogenic environment, although the exact roles of these pro-/antiangiogenic factors and how platelets regulate their release remain to be determined. Evidence suggests that these different factors maybe compartmentalized into separate platelet granules,57,58,104 or different granule proteins might be spatially packaged into distinct zones of the same granules,135 allowing them to be preferentially released upon different stimuli.136
Therefore, tumor-associated platelets may prefer a proangiogenic phenotype. Indeed, clinical studies have demonstrated that platelets from cancer patients have increased levels of VEGF, PDGF, PF4, angiopoietin-1, matrix metalloproteinase-2, and IL-6.105,120,137 The molecular mechanisms of phenotype changes remain largely unknown, but might be because tumor cells alter platelet transcriptome,63,138 or platelets actively sequester tumor-derived angiogenic proteins,139 which could then be delivered to the disseminated tumor sites.123,140 Platelets also prevent intratumoral hemorrhage and stabilize the tumor vessels via secreting angiopoietin-1 and 5-HT.141 Intriguingly, the antiangiogenic PF4 is significantly increased in platelets, but not in the plasma of tumor-bearing mice.142 Whether platelet-associated PF4 could preferentially inhibit intratumoral hemorrhage through binding to heparan sulfate at injured/immature angiogenic sites remains to be established.143 Altogether, these studies demonstrate that platelets facilitate cancer to sustain proliferative signaling, resist cell death, and induce angiogenesis.
Platelets activate tumor invasion and support metastasis
Platelets induce an invasive EMT phenotype of tumor cells and promote cell survival in blood circulation.
Cancer cell epithelial-mesenchymal transition (EMT) is considered a central mechanism by which transformed epithelial cells become more invasive.62 Platelet-treated tumor cells have a downregulated E-cadherin level, loss of which is considered to be a fundamental event in EMT,145 and an upregulated expression of mesenchymal markers, such as Snail, vimentin, fibronectin, and matrix metalloproteinase-9, and an increased prometastatic gene signature.146 Thus, platelets can promote tumor cell migration and invasion into the surrounding microenvironment. Moreover, the activation of the tumor invasion-metastasis cascade by platelets depends on the synergistic activation of both platelet-derived TGF-β/Smad and NF-κB pathways in cancer cells, which are triggered by direct platelet–tumor cell contact.146 Other platelet-released mediators have also been suggested to play a role in tumor EMT, such as prostaglandin (PG) E2, PDGF, and lysophosphatidic acid (LPA).147-151
The role of chemokine CCL5 in cancer invasion has been well recognized.152 Mesenchymal stem cells within tumor stroma secrete CCL5 that induces a tumor-invasive behavior via CCR5 on cancer cells.153 Anti-CCR5 therapy resulted in the repolarization of tumor-associated macrophages from protumor toward antitumor effects in patients with liver metastases.154 Because platelet-secreted CCL5 can induce monocyte and T-lymphocyte adhesion/transmigration,155,156 tumor-activated platelets may also release CCL5 to elicit tumor cell migration/invasion. In addition, a recent and elegant study identified a subpopulation of CD36+ metastasis-initiating cells in tumors.157 CD36 can drive metastasis by promoting fatty acid uptake and lipid metabolism.157 Because platelets also express abundant CD36,73 it is conceivable that platelets may transfer their CD36 to tumor cells and affect CD36-mediated metastasis. The observed antimetastatic effect of neutralizing anti-CD36 antibodies may result from their antiplatelet and/or tumor effects, including the potential inhibition of CD36-mediated platelet activation74-76 and/or CD36-thrombospondin-1–mediated21 TCIPA. These hypotheses remain to be examined.
After tumor cells detach from the primary site and intravasate into blood vessels, platelets are essential for tumor cell survival and transit in circulation.9 Experimental metastasis is almost completely abolished in nuclear factor erythroid-derived 2 knockout mice that have impaired platelet production.158 Platelets can rapidly associate with metastatic tumor cells via their receptors and cause TCIPA in circulation (see “TCIPA and formation of tumor-platelet aggregates”). Activated platelets can also provide procoagulant surfaces for cell-based thrombin generation,19 which further activates platelets, leukocytes, and tumor cells, enhancing TCIPA. It was previously considered that platelets might passively provide a “shield” for the circulating tumor cells. However, we now know that TCIPA is not only important to protect circulating tumor cells against shear-induced cell membrane damage in circulation,159 but is also an essential immune surveillance escape mechanism.11,160
Platelets facilitate tumor arrest at the endothelium, extravasation, and seeding.
The contribution of platelets to tumor arrest at the endothelium mainly involves adhesive interactions between platelets and endothelium, tumor cells, and leukocytes.9,11 First, tumor cells, or tumor-activated platelets, can induce endothelial activation by their soluble factors, EVs, and proteases.13,161 Activated endothelium can then directly recruit tumor cells, or platelet-tumor aggregates via several receptors, for instance, P-selectin, E-selectin, αVβ3 integrin, VWF, VCAM-1, and ICAM-1, and their ligands on tumor cells or platelets.11,87 Platelets, likely via the platelet-derived cytokines such as CCL5,56 engage monocytes to tumor cells and endothelium, which further enhances endothelial activation and indirectly facilitates tumor cell extravasation.162,163 Furthermore, platelet-derived CXCL5 and CXCL7 chemokines recruit granulocytes and guide the formation of the early metastatic niche.12 The formation of such cellular assemblies (ie, heteroaggregates of host–tumor cells) appears to be required for subsequent efficient metastasis.11
Moreover, available experimental evidence indicates that platelets can also directly enhance tumor extravasation.9,11,146 As noted previously, platelet-released TGF-β and the direct platelet–tumor contact synergistically promoted cancer EMT and successful extravasation.146 Additionally, platelet-derived LPA can support the progression of osteolytic bone metastases in breast cancer, likely involving the activation of the LPA receptor type 1 expressed on tumor cells.164,165 Furthermore, tumor-activated platelets can generate autotaxin, an LPA-producing enzyme, which interacts with tumor αVβ3 integrin and thus generates more LPA to support metastasis.150,151,166 It is currently unknown whether platelets could also support the adaptation of metastatic tumor cells to foreign tissue microenvironments and successful colonization, the last step of metastasis.62 Clarifying the potential roles of platelets in enabling metastatic colonization represents an important agenda for future research.
Platelets promote tumor evasion of immune destruction
The immune system plays key roles in tumor immunosurveillance. Tumor-infiltrating lymphocytes (TILs) correlate with the improved survival of patients with melanoma, breast, esophageal, colorectal, and ovarian cancers.167-170 However, surviving tumor cells turn to harness the immune system by hijacking its antitumor effects or attracting immunosuppressive cells.171-175 The past 3 decades have seen the successful discovery of novel cancer immunotherapy, such as immune checkpoint inhibitors, chimeric antigen receptor T-cell therapy and vaccine treatments.176
Platelets have been recognized as immune cells.6,55,56 However, their proinflammatory molecules, chemokines, and cytokines may facilitate not only inflammation and immune response but also TCIPA,6,55,56 which relates to malignancy.177 Interestingly, platelets may also be immune suppressive during tumorigenesis. It was previously found that platelets protected tumors from natural killer (NK) cell-mediated lysis in circulation178,179 (and likely also in the TME). Tumor-activated platelets release a large amount of TGF-β, which downregulated the expression of NKG2D, the major receptor on NK cells to sense stress-associated molecules such as major histocompatibility complex class I chain-related proteins A and B,180,181 impairing interferon-γ production and NK cell cytotoxicity.182 TGF-β also suppressed mTOR activity in NK cells, which inhibited NK cell activation/function.183 Additionally, platelets can transfer their major histocompatibility complex class I molecules to tumor cells,184 and tumor cells can also resemble platelets by displaying several platelet receptor markers.185 This “platelet mimicry” allows tumors to evade attacks from NK cells.184,185 Another mechanism may involve platelet glucocorticoid-induced tumor necrosis factor receptor ligand-mediated interference with NK cell immunosurveillance.186 Overall, these studies demonstrate that platelets protect tumor cells from NK cell–mediated lysis.
Tumor-associated platelets may also affect the activity of other immune cells through multiple receptors and a range of immunomodulatory chemokines, for instance, leukocyte trafficking.187,188 Recent evidence shows that metastatic breast cancer cells induce neutrophils to form metastasis-supporting neutrophil extracellular traps.189,190 It is possible that tumor-associated platelets may mediate neutrophil trafficking and extravasation,191 for example, via interactions between platelet GPIbα and neutrophil αMβ2 integrin,192 and may form tumor–platelet–neutrophil complexes to potentially enhance immune escape.
In addition, platelets may “paralyze” TIL by secreting large amounts of TGF-β, or by TGF-β delivered from platelet EV. Importantly, recent studies have provided some insights; glycoprotein A repetitions predominant (GARP), which is the cell surface docking receptor for latent TGF-β that causes TGF-β activation, was overexpressed in patients with breast, lung, and colon cancers. The TGF-β-GARP axis in the TME promotes T regulatory cell-mediated immune suppression.193 Most recently, experimental evidence showed that the majority of functional TGF-β is actually generated by platelets, both systemically and locally at the site of tumor, because platelets also express GARP receptor, rather than secrete TGF-β alone.194 Furthermore, a combination of antiplatelet agents (eg, aspirin and clopidogrel) markedly improves the efficacy of adoptive T-cell therapy against cancer in animal models.194 These data indicate that platelets are able to directly subvert T-cell immunity. Interestingly, evidence emerged suggesting platelets and platelet-derived microparticles can also infiltrate tumors118,195 ; it is currently unclear whether they can directly interact with TIL. These questions remain to be addressed.
Based on the intensive tumor–platelet interactions, other applications have also been suggested. For example, conjugation of platelets to the anti-programmed death-ligand 1 antibody facilitates the delivery of anti-programmed death-ligand 1 to the site of postsurgical residual microtumors and circulating tumor cells, thus reducing postsurgical tumor recurrence and experimental metastasis.196 Altogether, platelets may have “carcinogenic” potential and thrombocytosis may facilitate malignancy, which might therefore be a desirable target for cancer therapy.
Aspirin protects against cancer
Since its first synthesis in 1897, aspirin, a nonsteroidal anti-inflammatory drug, has been one of the most widely used medications to reduce pain, fever, inflammation, and platelet activity. Mounting evidence has supported its new use in cancer prevention, reducing metastasis and mortality, especially for colorectal cancer (CRC).3,197-204 In 2016, the US Preventive Services Task Force incorporated the prophylactic effect of low-dose aspirin on CRC and recommends the initiation of daily low-dose aspirin (eg, 75-100 mg/day) for at least 10 years in adults aged 50 to 69 years with specific CVD risk.5 In contrast, considering the risks (eg, bleeding tendency, gastrointestinal disorders) vs unproven benefits of long-term aspirin use, the European Guidelines in clinical practice do not support its role in primary prevention.205,206 However, some investigators believe that low-dose aspirin does not significantly cause bleeding complications in average-risk individuals.206-209
Other evidence suggests that aspirin might also reduce the incidence of nongastrointestinal cancers, such as cholangiocarcinoma, breast, prostate, lung, endometrial, pancreatic, and ovarian cancers.210-217 However, these findings should be interpreted with caution because of study heterogeneity, and the clinical data are not always consistently promising.197,202,211 Large randomized controlled trials to further determine its contribution are warranted.
Platelet-mediated mechanism of aspirin
Aspirin is an irreversible cyclooxygenase (COX) inhibitor through acetylation of a serine residue that reduces the synthesis of prostanoids, such as PGE2 and TXA2, from arachidonic acid. COX-1 is constitutively expressed in platelets and gastric epithelial cells and is responsible for the generation of TXA2 in platelets and the basal production of cytoprotective prostaglandins in the gastric mucosa. COX-2 is not normally expressed in most cells (except some tissues such as endothelium); however, it is progressively overexpressed in many cancer cells, including colorectal, breast, gastric, lung, and pancreatic cancers and melanoma. A critical event in tumorigenesis and metastasis involves the enhanced synthesis of PGE2 by COX, which in turn enhances tumor proliferation, angiogenesis, differentiation, inflammation, and immune escape.218-220 The capacity of aspirin to inhibit COX activity and prostanoid production has been widely considered the central mechanism of its anticancer effects; however, a significant gap occurs when determining how much of the effect is platelet-dependent and how much is owed to the direct inhibition of COX in other cells, such as tumor cells.
It was considered that COX-2 inhibition by aspirin is essential for its anticancer action. Aspirin use was found to correlate with reduced risk of CRC in patients that overexpressed COX-2, but not in those who had weak or absent COX-2 expression.221 However, subsequent clinical and pharmacology studies suggest that the antiplatelet effect of aspirin (ie, permanent inactivation of platelet COX-1) is sufficient and necessary for its anticancer action.3,198-200,202 First, similar effects of aspirin at doses of 75 to 300 mg/day have been shown to reduce cancer incidence, metastasis, and mortality, with 75 mg/day being as effective as higher doses; and dosing at 24-hour intervals appears to be sufficient.198-200,202 Moreover, chemopreventive effects were found with a low-dose, slow-release formulation of aspirin that was designed to specifically inhibit platelet function with few systemic effects.200 Also, aspirin has a short half-life (20 minutes) in human circulation. Daily low-dose aspirin is able to irreversibly and completely inhibit platelet COX-1 activity and TXA2 production; consequently, the profound inhibition of platelet function (eg, TCIPA) by aspirin persists throughout the dose interval (ie, 24 hours). In contrast, this dose cannot achieve sustained inhibition of COX-2 in nucleated cells because nucleated cells have the capacity to de novo synthesize COX isozymes within a few hours, whereas platelets cannot.222 Higher doses of aspirin (eg, 650 mg 3 times/day) have been shown to be required for sustained inhibition of COX-2.202,223
Experimental evidence with aspirin also demonstrates a platelet-related mechanism. Aspirin inhibited platelet-induced angiogenesis after exposure to breast cancer cells,104 reduced platelet-promoted colon and pancreatic cancer cell proliferation,224 and rescued platelet-accelerated metastatic potential of colon cancer cells, which is likely through inhibiting platelet COX-1–mediated TXA2 and PGE2 biosynthesis.149 Moreover, aspirin plus clopidogrel improved the efficacy of adoptive T-cell therapy against cancer194 and prevented hepatitis B virus–associated liver cancer in animal models.225 Combined, these findings underscore a platelet-dependent effect of low-dose aspirin in cancer prevention.226,227 Because platelets play important roles in tumorigenesis and metastasis, platelet inhibition may bring greater benefits than once thought.
Intriguingly, the blockade of platelet COX-1 activity may also negatively regulate the expression and function of COX-2 in adjacent cancer cells.3,202,228 Studies showed that platelets induced overexpression of COX-2 in colon carcinoma cells through direct platelet–cancer cell interaction and release of paracrine lipid and protein mediators.148,208 Platelet-derived Wnt caused β-catenin translocation into the nucleus and the rapid increase of COX-2 mRNA in cancer cells.227 Aspirin could inhibit platelet activation and platelet-mediated COX-2 expression in adjacent nucleated cells at sites of mucosal injury.229,230 However, deciphering the crosstalk between COX-1 and COX-2 in different cells during cancer development is a challenging question to be further investigated.
COX-independent mechanisms of aspirin in cancer have also been suggested, including modifications of NF-κB and RUNX1,231 induction of cancer cell apoptosis, reversal of hypermethylation of tumor suppressor genes, downregulation of mutation-inducing DNA damage, and acetylation of intracellular RNA.232-234 However, most of these effects have been characterized in vitro using supratherapeutic concentrations of aspirin, and the in vivo effects and evidence by low-dose aspirin is lacking.
Opportunities and challenges for other antiplatelet agents
As mentioned for antiplatelet agents,99 aspirin and several COX-1 inhibitors have been used in patients for decades to centuries. Integrin αIIbβ3 antagonists and adenosine 5′-diphosphate receptor (P2Y12) antagonist clopidogrel were prescribed to patients in the 1990s. Several newer P2Y12 antagonists and a thrombin receptor (PAR1) antagonist235 have also been recently approved by US Food and Drug Administration. Although aside from aspirin, little clinical information is available regarding other antiplatelet agents in cancer81 ; it is predictable that some of them may be beneficial for patients.79-81,194,225,235,236
Several other antiplatelet drugs are in the preclinical stage or different phases of clinical trials, and some may be available in the market in the near future.237 Besides P-selectin inhibitors, GPVI and GPIbα antagonists are under development.99 Although GPVI was considered as the platelet activation receptor for fibrillar collagen on subendothelial matrix, a recent study demonstrated that it bound to fibrin(ogen),238,239 leading to the possible involvement of GPVI in TCIPA, which may explain its supportive role in metastasis in mice.240 It will be interesting to reexamine whether GPVI antagonists have confounding effects on tumor metastasis.241 GPIbα antagonists should be highlighted because anti-GPIbα may have dual roles in metastasis.91,94 As reported by us and others, antibodies against GPIbα inhibited not only VWF binding, but also its interactions with thrombin95 and other molecules such as αMβ2 integrin192 and P-selectin,242 which may have broad inhibitory effects on TCIPA and tumor–platelet–leukocyte heterotypical aggregation. Additionally, as we recently observed, anti-GPIbα antibodies can decrease thrombopoietin generation,119 which may inhibit tumor-induced thrombocytosis. Other emerging antiplatelet agents include those targeting platelet-activating receptors (eg, anti-CD36,157 anti-CLEC 2,71,72 EP3 receptor antagonists149,243 ) and inhibitory receptors (eg, anti-PECAM-1, CEACAM1),70,244 etc. Furthermore, other agents of platelet blockade, such as anticoagulants that inhibit thrombin-induced platelet activation/TCIPA, non-aspirin nonsteroidal anti-inflammatory drugs and plant-based food products (eg, anthocyanins) may also have antitumor effects.245-247 Characterizing these new agents will certainly advance our knowledge and treatment to control cancer (Table 1).
Although we focus here on “how cancer changes platelets to be more cancer-friendly,” one cannot exclude the potential “dual” (ie, supportive and inhibitive) roles of platelets in tumor progression. An elegant study recently showed that platelet microparticles can infiltrate solid tumors and deliver miR-24, which induces tumor cell apoptosis and suppresses tumor growth.190,195 In fact, platelets are versatile and part of the innate immune system. They can modify adaptive immunity and therefore may significantly contribute to immunosurveillance.53,55,56 Furthermore, although the prevailing view is that platelets are proinflammatory and immune supportive, our recent data unveiled their immune-suppressive activities following platelet desialylation.248 Also, platelets contain both pro- and antiangiogenic factors. We therefore cannot exclude that different antiplatelet drugs, the same drug in different doses or patients may have different consequences or even detrimental effects. A better understanding of the pro- and antitumor activities of platelets could be the next big breakthrough that will advance our knowledge in platelet–cancer interactions for therapeutic benefits. More basic and clinical studies should be able to address these questions.
Summary
This article highlights evidence for intimate crosstalk between cancer and platelets (Figure 1). Tumor-associated platelet proteins, RNA profiles, and thrombocytosis may be useful biomarkers for cancer screening, diagnosis, prognosis, and treatment monitoring. Further clinical trials are needed to identify and validate cancers that are closely linked with these signatures. In addition, TEP-based liquid biopsy assay is emerging, although further characterization is required before it can be a reliable diagnostic tool. Reciprocally, platelets can further support tumorigenesis and metastasis. Targeting platelet–cancer crosstalk may represent a novel and promising antitumor strategy. Several prospective clinical trials are currently evaluating the benefits of adjuvant aspirin treatment in patients with colorectal, breast, esophageal, ovarian, or lung cancers. Notably, however, the role of chronic platelet inhibition in cancers is not always consistent. Dual antiplatelet therapy by prasugrel, ticagrelor, or vorapaxar on top of aspirin was shown to correlate with excess tumor growth and cancer-associated death in several clinical trials.249-252 The exact mechanisms are still unclear, but it might be due to the impairment of the possible antitumor activity of platelets.195,253
The dynamic requisites of tumor cells during tumorigenesis and metastasis have given rise to challenging questions to fully understand the exact roles of platelets at different stages of cancer; how platelets may balance their pro- and antitumor activities, which might involve distinct signaling pathways and molecule variants; and why platelet inhibition by aspirin works best in certain cancers. Furthermore, to identify individuals for whom the benefits outweigh the hazards (eg, hemorrhage, thrombocytopenia, gastrointestinal disorders, immune alterations) and determine sensitive tumor types for antiplatelet treatment are of great importance for personalized medicine. Other challenges such as the requirement for intravenous infusion of some antiplatelet agents (eg, αIIbβ3 antagonists) may add difficulties to the clinical trials. Nonetheless, adjuvant treatment with aspirin and other antiplatelet agents may open a new era and opportunity for antitumor therapy.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Reid C. Gallant, Brigitta Elaine Oswald, Xun Fu, and Tyler W. Stratton for editing the manuscript.
This work was supported in part by a grant-in-aid from the Heart and Stroke Foundation of Canada (Ottawa, ON, Canada), Canadian Institutes of Health Research (CIHR) (Open Operating Grants Program [MOP] 119540, MOP 97918, MOP 68986, and MOP 119551), and CIHR–Canadian Blood Services Partnership.
Authorship
Contribution: All authors contributed to the preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Heyu Ni, Department of Laboratory Medicine and Pathobiology, Department of Medicine, and Department of Physiology, University of Toronto, Scientist of Canadian Blood Services Centre for Innovation, Platform Director for Hematology, Cancer and Immunological Diseases, St. Michael's Hospital, Room 420, LKSKI–Keenan Research Centre, 209 Victoria St, Toronto, ON M5B 1W8, Canada; e-mail: nih@smh.ca.